LAMICTAL Dispersible / Chewable tablet Ref.[50798] Active ingredients: Lamotrigine
Source: Medicines and Medical Devices Safety Authority (NZ) Revision Year: 2021 Publisher: GlaxoSmithKline NZ Limited, Private Bag 106600, Downtown, Auckland, NEW ZEALAND, Phone: (09) 367 2900, Facsimile: (09) 367 2910
4.1. Therapeutic indications
Epilepsy
Adults and Adolescents (over 12 years of age)
LAMICTAL is indicated as adjunctive therapy in the treatment of epilepsy, for partial seizures and generalised seizures, including tonic-clonic seizures and the seizures associated with Lennox-Gastaut syndrome.
Children (2 to 12 years of age)
LAMICTAL is indicated as adjunctive therapy in the treatment of epilepsy, for partial seizures and generalised seizures including tonic-clonic seizures and the seizures associated with Lennox-Gastaut syndrome.
Bipolar Disorder (Adults 18 years of age and over)
LAMICTAL is indicated for the prevention of mood episodes in patients with bipolar disorder, predominantly by preventing depressive episodes.
4.2. Posology and method of administration
Dose
Restarting Therapy
Prescribers should assess the need for escalation to maintenance dose when restarting lamotrigine in patients who have discontinued lamotrigine for any reason, since the risk of serious rash is associated with high initial doses and exceeding the recommended dose escalation for lamotrigine (see section 4.4 Special warnings and precautions for use). The greater the interval of time since the previous dose, the more consideration should be given to escalation to the maintenance dose. When the interval since discontinuing lamotrigine exceeds five half-lives (see section 5.2 Pharmacokinetic properties), lamotrigine should generally be escalated to the maintenance dose according to the appropriate schedule. It is recommended that lamotrigine not be restarted in patients who have discontinued due to rash associated with prior treatment with lamotrigine unless the potential benefit clearly outweighs the risk.
EPILEPSY
Dosage in EPILEPSY add-on therapy
Adults and Adolescents (over 12 years of age):
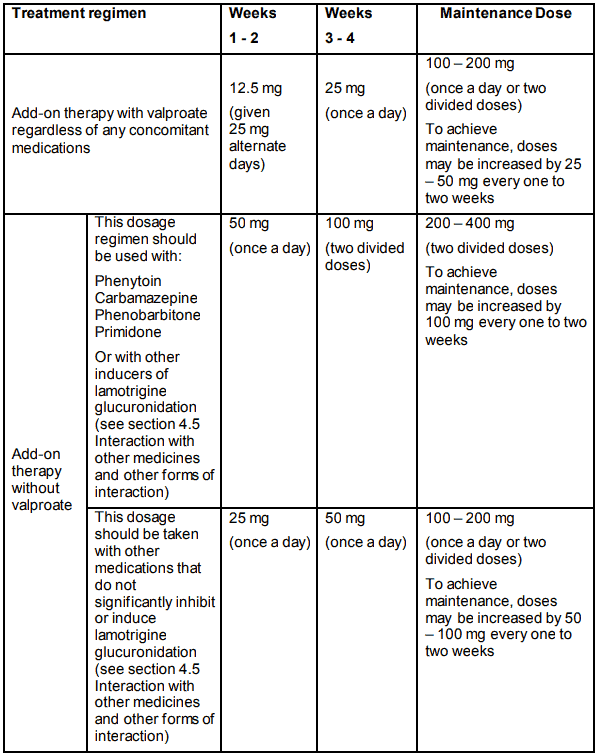
In patients taking valproate with/without any other anti-epileptic medicine (AEM), the initial lamotrigine dose is 25 mg every alternate day for two weeks, followed by 25 mg once a day for two weeks. Thereafter, the dose should be increased by a maximum of 25-50 mg every 1-2 weeks until the optimal response is achieved. The usual maintenance dose to achieve optimal response is 100-200 mg/day given once a day or in two divided doses.
In those patients taking concomitant AEMs or other medications (see section 4.5 Interaction with other medicines and other forms of interaction) that induce lamotrigine glucuronidation with/without other AEMs (except valproate), the initial lamotrigine dose is 50 mg once a day for two weeks, followed by 100 mg/day given in two divided doses for two weeks.
Thereafter, the dose should be increased by a maximum of 100 mg every 1-2 weeks until the optimal response is achieved. The usual maintenance dose to achieve optimal response is 200-400 mg/day given in two divided doses.
Some patients have required 700 mg/day of lamotrigine to achieve the desired response.
In patients taking other medications that do not significantly inhibit or induce lamotrigine glucuronidation (see section 4.5 Interaction with other medicines and other forms of interaction), the initial lamotrigine dose is 25 mg once a day for two weeks, followed by 50 mg once a day for two weeks. Thereafter, the dose should be increased by a maximum of 50 to 100 mg every one to two weeks until the optimal response is achieved. The usual maintenance dose to achieve optimal response is 100 to 200 mg/day given once a day or as two divided doses.
Recommended treatment regimen in EPILEPSY for adults and adolescents over 12 years of age:
In patients taking anti-epileptic medicines where the pharmacokinetic interaction with lamotrigine is currently not known (see section 4.5 Interaction with other medicines and other forms of interaction), the treatment regimen as recommended for lamotrigine with concurrent valproate should be used.
Because of a risk of rash the initial dose and subsequent dose escalation should not be exceeded (see section 4.4 Special warnings and precautions for use).
Children (2 to 12 years of age):
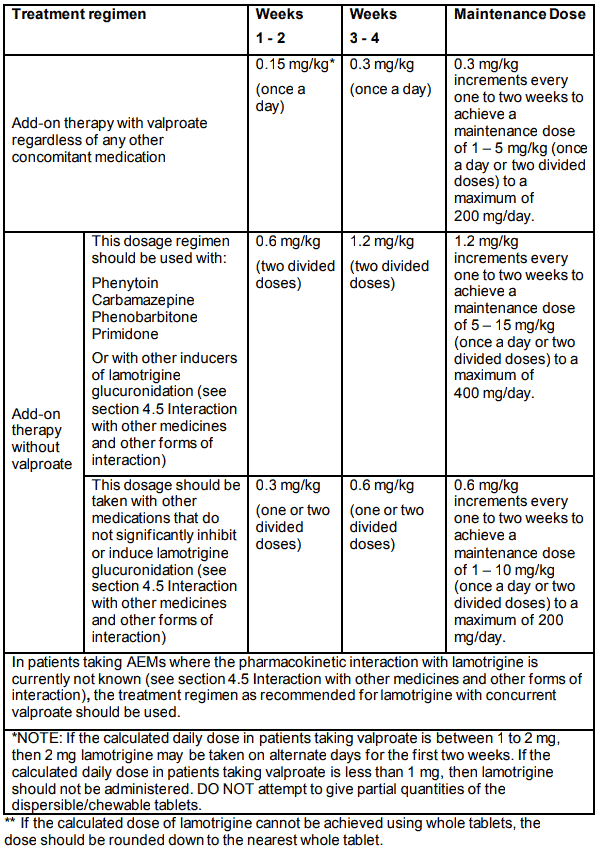
In patients taking valproate with/without any other AEM, the initial lamotrigine dose is 0.15 mg/kg bodyweight/day given once a day for two weeks, followed by 0.3 mg/kg/day once a day for two weeks. Thereafter, the dose should be increased by a maximum of 0.3 mg/kg every 1-2 weeks until the optimal response is achieved. The usual maintenance dose to achieve optimal response is 1-5 mg/kg/day given once a day or in two divided doses, with a maximum of 200 mg/day.
In those patients taking concomitant AEMs or other medications (see section 4.5 Interaction with other medicines and other forms of interaction) that induce lamotrigine glucuronidation with/without other AEMs (except valproate), the initial lamotrigine dose is 0.6 mg/kg bodyweight/day given in two divided doses for two weeks, followed by 1.2 mg/kg/day given in two divided doses for two weeks. Thereafter, the dose should be increased by a maximum of 1.2 mg/kg every 1-2 weeks until the optimal response is achieved. The usual maintenance dose to achieve optimal response is 5-15 mg/kg/day given once a day or in two divided doses, with a maximum of 400 mg/day.
In patients taking other medications that do not significantly inhibit or induce lamotrigine glucuronidation (see section 4.5 Interaction with other medicines and other forms of interaction), the initial lamotrigine dose is 0.3 mg/kg bodyweight/day given once a day or in two divided doses for two weeks, followed by 0.6 mg/kg/day given once a day or in two divided doses for two weeks. Thereafter, the dose should be increased by a maximum of 0.6 mg/kg every one to two weeks until the optimal response is achieved. The usual maintenance dose to achieve optimal response is 1 to 10 mg/kg/day given once a day or in two divided doses, with a maximum of 200 mg/day.
To ensure a therapeutic dose is maintained the weight of a child must be monitored and the dose reviewed as weight changes occur.
Recommended treatment regimen in EPILEPSY for children aged 2-12 years (total daily dose in mg/kg bodyweight/day)**:
Because of a risk of rash, the initial dose and subsequent dose escalation should not be exceeded (see section 4.4 Special warnings and precautions for use).
It is likely that patients aged 2-6 years will require a maintenance dose at the higher end of the recommended range.
Children aged less than 2 years:
Lamotrigine has not been studied as monotherapy in children less than 2 years of age or as add-on therapy in children less than 1 month of age. The safety and efficacy of lamotrigine as add-on therapy of partial seizures in children aged 1 month to 2 years has not been established (see section 5 Pharmacological properties). Therefore, lamotrigine is not recommended in children less than 2 years of age.
General Dosing Recommendations for EPILEPSY
When other anti-epileptic medicines (AEMs) are added-on to treatment regimes containing lamotrigine, consideration should be given to the effect this may have on lamotrigine pharmacokinetics (see section 4.5 Interaction with other medicines and other forms of interaction).
BIPOLAR DISORDER
Adults (18 years of age and over)
Because of the risk of rash, the initial dose and subsequent dose escalation should not be exceeded (see section 4.4 Special warnings and precautions for use).
Lamotrigine is recommended for use in bipolar patients at risk for a future depressive episode.
The following transition regimen should be followed to prevent recurrence of depressive episodes. The transition regimen involves escalating the dose of lamotrigine to a maintenance stabilisation dose over six weeks after which other psychotropic and/or anti-epileptic medicines can be withdrawn, if clinically indicated.
Adjunctive therapy should be considered for the prevention of manic episodes, as efficacy with lamotrigine in mania has not been conclusively established.
Recommended dose escalation to the maintenance total daily stabilisation dose for adults (over 18 years of age) treated for BIPOLAR DISORDER:
a) Adjunct therapy with inhibitors of lamotrigine glucuronidation e.g. Valproate
In patients taking glucuronidation inhibiting concomitant medicines such as valproate the initial lamotrigine dose is 25 mg every alternate day for two weeks, followed by 25 mg once a day for two weeks. The dose should be increased to 50 mg once a day (or in two divided doses) in week 5. The usual target dose to achieve optimal response is 100 mg/day given once a day or in two divided doses. However, the dose can be increased to a maximum daily dose of 200 mg, depending on clinical response.
b) Adjunct therapy with inducers of lamotrigine glucuronidation in patients NOT taking inhibitors such as Valproate. This dosage regiment should be used with phenytoin, carbamazepine, phenobarbitone, primidone and other medicines known to induce lamotrigine glucuronidation (see section 4.5 Interaction with other medicines and other forms of interaction)
In those patients currently taking medicines that induce lamotrigine glucuronidation and NOT taking valproate, the initial lamotrigine dose is 50 mg once a day for two weeks, followed by 100 mg/day given in two divided doses for two weeks. The dose should be increased to 200 mg/day given as two divided doses in week 5. The dose may be increased in week 6 to 300 mg/day however, the usual target dose to achieve optimal response is 400 mg/day given in two divided doses which may be given from week 7.
c) Adjunct therapy in patients taking other medications that do not significantly induce or inhibit lamotrigine glucuronidation (see section 4.5 Interaction with other medicines and other forms of interaction)
The initial lamotrigine dose is 25 mg once a day for two weeks, followed by 50 mg once a day (or in two divided doses) for two weeks. The dose should be increased to 100 mg/day in week 5. The usual target dose to achieve optimal response is 200 mg/day given once a day or as two divided doses. However, a range of 100-400 mg was used in clinical trials.
Once the target daily maintenance stabilisation dose has been achieved, other psychotropic medications may be withdrawn as laid out in the dosage schedule below.
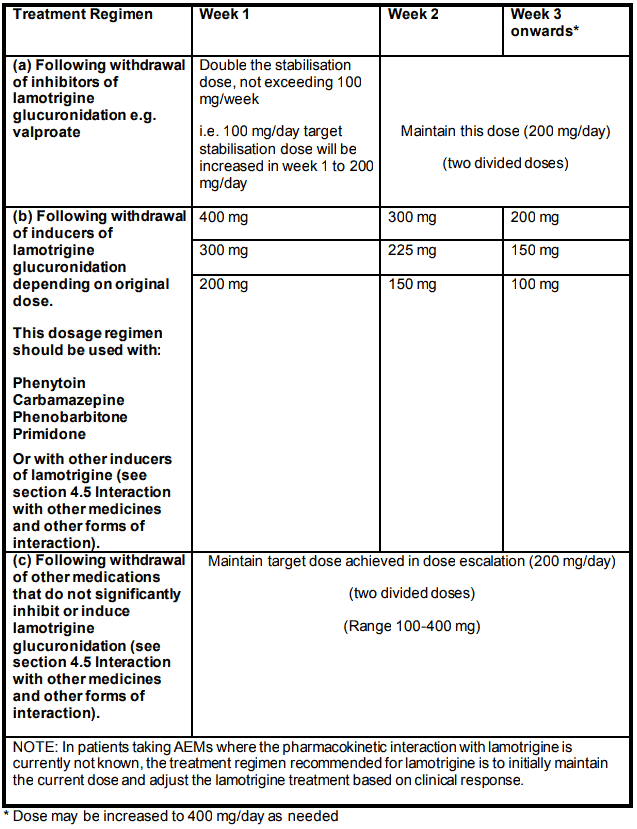
Maintenance stabilisation total daily dose in BIPOLAR DISORDER following withdrawal of concomitant psychotropic or anti-epileptic medicines:
(a) Following withdrawal of adjunct therapy with inhibitors of lamotrigine glucuronidation e.g. valproate
The dose of lamotrigine should be increased to double the original target stabilisation dose and maintained at this, once valproate has been terminated.
(b) Following withdrawal of adjunct therapy with inducers of lamotrigine glucuronidation depending on original maintenance dose. This regimen should be used with phenytoin, carbamazepine, phenobarbitone, primidone or other medicines known to induce lamotrigine glucuronidation (see section 4.5 Interaction with other medicines and other forms of interaction)
The dose of lamotrigine should be gradually reduced over 3 weeks as the glucuronidation inducer is withdrawn.
(c) Following withdrawal of adjunct therapy with other medications that do not significantly inhibit or induce lamotrigine glucuronidation (see section 4.5 Interaction with other medicines and other forms of interaction)
The target dose achieved in the dose escalation programme should be maintained throughout withdrawal of the other medication.
Adjustment of lamotrigine daily dosing in patients with BIPOLAR DISORDER following addition of other medications:
There is no clinical experience in adjusting the lamotrigine daily dose following the addition of other medications. However, based on medicine interaction studies, the following recommendations can be made:
Adjustment of lamotrigine daily dosing in patients with BIPOLAR DISORDER following the addition of other medications
Discontinuation of lamotrigine in patients with BIPOLAR DISORDER
In clinical trials, there was no increase in the incidence, severity or type of adverse experiences following abrupt termination of lamotrigine versus placebo. Therefore, patients may terminate lamotrigine without a step-wise reduction of dose.
Children and Adolescents (less than 18 years of age)
Lamotrigine is not indicated for use in bipolar disorder in children and adolescents aged less than 18 years (see section 4.4 Special warnings and precautions for use). Safety and efficacy of lamotrigine in bipolar disorder has not been evaluated in this age group. Therefore, a dosage recommendation cannot be made.
Special Populations
Elderly population (over 65 years of age)
No dosage adjustment from recommended schedule is required. The pharmacokinetics of lamotrigine in this age group do not differ significantly from a non-elderly adult population.
Renal impairment
Caution should be exercised when administering lamotrigine to patients with renal failure. For patients with end-stage renal failure, initial doses of lamotrigine should be based on patients' AEM regimen; reduced maintenance doses may be effective for patients with significant renal functional impairment (see section 4.4 Special warnings and precautions for use). For more detailed pharmacokinetic information see section 5.2 Pharmacokinetic properties.
Hepatic impairment
Initial, escalation and maintenance doses should generally be reduced by approximately 50% in patients with moderate (Child-Pugh grade B) and 75% in severe (Child-Pugh grade C) hepatic impairment. Escalation and maintenance doses should be adjusted according to clinical response (see section 5.2 Pharmacokinetic properties).
Women taking hormonal contraceptives
(a) Starting lamotrigine in patients already taking hormonal contraceptives:
Although an oral contraceptive has been shown to increase the clearance of lamotrigine (see section 4.4 Special warnings and precautions for use and section 4.5 Interaction with other medicines and other forms of interaction), no adjustments to the recommended dose escalation guidelines for lamotrigine should be necessary solely based on the use of hormonal contraceptives. Dose escalation should follow the recommended guidelines based on whether lamotrigine is added to valproate (an inhibitor of lamotrigine glucuronidation) or to an inducer of lamotrigine glucuronidation, or whether lamotrigine is added in the absence of valproate or an inducer of lamotrigine glucuronidation.
(b) Starting hormonal contraceptives in patients already taking maintenance doses of lamotrigine and NOT taking inducers of lamotrigine glucuronidation:
The maintenance dose of lamotrigine will in most cases need to be increased by as much as two-fold (see section 4.4 Special warnings and precautions for use and section 4.5 Interaction with other medicines and other forms of interaction). It is recommended that from the time that the hormonal contraceptive is started, the lamotrigine dose is increased by 50 to 100 mg/day every week, according to the individual clinical response. Dose increases should not exceed this rate, unless the clinical response supports larger increases.
(c) Stopping hormonal contraceptives in patients already taking maintenance doses of lamotrigine and NOT taking inducers of lamotrigine glucuronidation:
The maintenance dose of lamotrigine will in most cases need to be decreased by as much as 50% (see section 4.4 Special warnings and precautions for use and section 4.5 Interaction with other medicines and other forms of interaction). It is recommended to gradually decrease the daily dose of lamotrigine by 50 to 100 mg each week (at a rate not exceeding 25% of the total daily dose per week) over a period of 3 weeks, unless the clinical response indicates otherwise.
Use with atazanavir/ritonavir
Although atazanavir/ritonavir has been shown to reduce lamotrigine plasma concentrations (see section 4.5 Interaction with other medicines and other forms of interaction), no adjustments to the recommended dose escalation guidelines for lamotrigine should be necessary solely based on the use of atazanavir/ritonavir. Dose escalation should follow the recommended guidelines based on whether lamotrigine is added to valproate (an inhibitor of lamotrigine glucuronidation), or to an inducer of lamotrigine glucuronidation, or whether lamotrigine is added in the absence of valproate or an inducer of lamotrigine glucuronidation.
In patients already taking maintenance doses of lamotrigine and not taking glucuronidation inducers, the lamotrigine dose may need to be increased if atazanavir/ritonavir is added, or decreased if atazanavir/ritonavir is discontinued.
Method of administration
LAMICTAL tablets may be chewed, dispersed in a small volume of water (at least enough to cover the whole tablet) or swallowed whole with a little water.
DO NOT attempt to administer partial quantities of the dispersible/chewable tablets. If a calculated dose of lamotrigine (e.g. for use in children (epilepsy only) or patients with hepatic impairment) cannot be divided into multiple lower strength tablets, the dose to be administered is that equal to the nearest lower strength of whole tablets.
4.9. Overdose
Symptoms and signs
Acute ingestion of doses in excess of 10-20 times the maximum therapeutic dose of LAMICTAL have been reported, including fatal cases. Overdose has resulted in symptoms including nystagmus, ataxia, impaired consciousness, grand mal convulsion and coma. QRS broadening (intraventricular conduction delay) has also been observed in overdose patients.
Treatment
In the event of overdosage, the patient should be admitted to hospital and given appropriate supportive therapy as clinically indicated or as recommended by the national poisons centre, where available.
For advice on the management of overdose please contact the National Poisons Centre on 0800 POISON (0800 764766).
6.3. Shelf life
2 years: 2 mg tablet
3 years: 5 mg, 25 mg, 50 mg and 100 mg tablets
6.4. Special precautions for storage
Do not store above 30°C. Keep dry. Protect from light.
6.5. Nature and contents of container
25 mg, 50 mg, 100 mg:
Blister packs of 56 tablets
Child resistant PVC/PVdC/aluminium foil/paper blister.
5 mg:
Blister packs of 28 or 30 tablets
PVC/PVdC/aluminium foil blister.
HDPE bottle packs of 30 tablets
HDPE bottles with a child resistant/tamper evident or
continuous thread closure.
2 mg:
HDPE bottle packs of 30 tablets
HDPE bottles with a child resistance/tamper evident closure.
Not all strengths, packs sizes or container types may be distributed in New Zealand.
6.6. Special precautions for disposal and other handling
No special requirements for disposal.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.