LAVENTAIR Inhalation powder, pre-dispensed Ref.[8299] Active ingredients: Umeclidinium Vilanterol
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: GlaxoSmithKline (Ireland) Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland
Pharmacodynamic properties
Pharmacotherapeutic group: Drugs for obstructive airway diseases, adrenergics in combination with anticholinergics incl. triple combinations with corticosteroids
ATC code: R03AL03
Mechanism of action
Umeclidinium/vilanterol is a combination inhaled long-acting muscarinic receptor antagonist/long-acting beta2-adrenergic agonist (LAMA/LABA). Following oral inhalation both compounds act locally on airways to produce bronchodilation by separate mechanisms.
Umeclidinium
Umeclidinium is a long acting muscarinic receptor antagonist (also referred to as an anticholinergic). It is a quinuclidine derivative with activity across multiple muscarinic receptor subtypes. Umeclidinium exerts its bronchodilatory activity by competitively inhibiting the binding of acetylcholine with muscarinic receptors on airway smooth muscle. It demonstrates slow reversibility at the human M3 muscarinic receptor subtype in vitro and a long duration of action in vivo when administered directly to the lungs in pre-clinical models.
Vilanterol
Vilanterol is a selective long-acting, beta2-adrenergic receptor agonist (beta2-adrenergic agonist). The pharmacologic effects of beta2-adrenergic agonists, including vilanterol, are at least in part attributable to stimulation of intracellular adenylate cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3',5'-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
Pharmacodynamic effects
In Phase III, 6-month studies umeclidinium/vilanterol provided clinically meaningful improvements over placebo in lung function (as measured by forced expiratory volume in 1 second [FEV1]) over 24 hours following once daily administration, which were evident at 15 minutes following administration of the first dose (improvement over placebo by 112 ml (p<0.001*). Mean peak improvements in FEV1 within the first 6 hours following dosing relative to placebo was 224 ml (p<0.001*) at Week 24. There was no evidence for tachyphylaxis in the effect of LAVENTAIR ELLIPTA over time.
Cardiac electrophysiology
The effect of umeclidinium/vilanterol on the QT interval was evaluated in a placebo and active (moxifloxacin) controlled QT study involving once daily administration of umeclidinium/vilanterol 113/22 micrograms or 500/100 micrograms (pre-dispensed dose with umeclidinium at eight times the recommended dose and vilanterol at four times the recommended dose) for 10 days in 103 healthy volunteers. The maximum mean difference in prolongations of QT interval (corrected using the Fridericia method, QTcF) from placebo after baseline-correction was 4.3 (90% CI=2.2 to 6.4) milliseconds seen 10 minutes after administration with umeclidinium/vilanterol 113/22 micrograms and 8.2 (90% CI=6.2 to 10.2) milliseconds seen 30 minutes after administration with umeclidinium/vilanterol 500/100 micrograms. Therefore, no clinically relevant pro-arrhythmic potential related to QT-interval prolongations was observed with umeclidinium/vilanterol 113/22 micrograms.
A dose-dependent increase in heart rate was also observed. The maximum mean difference in heart rate from placebo after baseline-correction was 8.4 (90% CI=7.0 to 9.8) beats/minute and 20.3 (90% CI=18.9 to 21.7) beats/minute seen 10 minutes after administration of umeclidinium/vilanterol 113/22 micrograms and 500/100 micrograms respectively.
In addition, no clinically significant effects on cardiac rhythm were observed on 24-hour Holter monitoring in 53 patients with COPD who were treated with umeclidinium/vilanterol 55/22 micrograms once daily in one 6-month study, or in a further 55 patients who received umeclidinium/vilanterol 113/22 micrograms once daily in another 6-month study and 226 patients who received 113/22 micrograms once daily in the 12-month study.
* A step-down statistical testing procedure was used in this study and this comparison was below a comparison that did not achieve statistical significance. Therefore, statistical significance on this comparison cannot be inferred.
Clinical efficacy and safety
The clinical efficacy of umeclidinium/vilanterol administered once daily was evaluated in eight Phase III clinical studies in 6,835 adult patients with a clinical diagnosis of COPD; 5,618 patients from five 6-month studies (two placebo-controlled and three active [tiotropium]-comparator controlled), 655 patients from two 3-month exercise endurance/lung function studies and 562 patients from a 12-month supportive study.
Effects on lung function
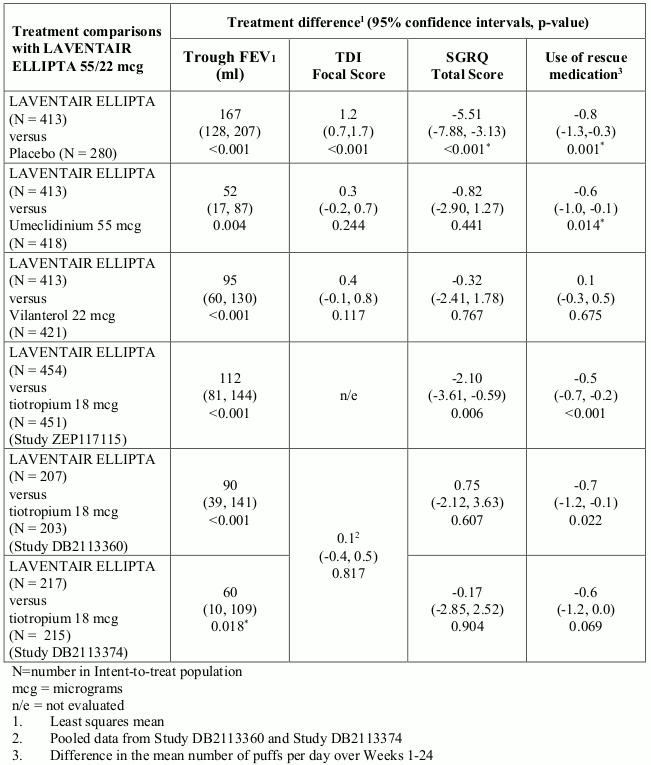
LAVENTAIR ELLIPTA demonstrated improvements in lung function (as defined by change from baseline in trough FEV1) in several studies. In one 6-month Phase III study, LAVENTAIR ELLIPTA demonstrated statistically significant improvements in trough FEV1 (primary endpoint) at Week 24 compared with placebo and each monotherapy component treatment arm. In addition, LAVENTAIR ELLIPTA demonstrated clinically meaningful and statistically significant improvements in trough FEV1 compared with tiotropium in two of the three 6-month active-comparator studies and numerically greater improvements from tiotropium in the third active-comparator study (see Table 1). There was no attenuation of the bronchodilator effect over time.
Symptomatic outcomes
Breathlessness
LAVENTAIR ELLIPTA demonstrated a statistically significant and clinically meaningful reduction in breathlessness as evaluated by an increase in TDI focal score at Week 24 (key secondary end-point) compared with placebo (see Table 1). Improvements in TDI focal score compared with each monotherapy component and tiotropium were not statistically significant (see Table 1).
The proportion of patients who responded with at least the minimum clinically important difference (MCID) of 1 unit TDI focal score at Week 24 was greater for LAVENTAIR ELLIPTA (58%) compared with placebo (41%) and each monotherapy component (53% for umeclidinium and 51% for vilanterol).
Health-related quality of life
LAVENTAIR ELLIPTA has also shown an improvement in health-related quality of life measured using the St. George’s Respiratory Questionnaire (SGRQ) as indicated by a reduction in SGRQ total score at Week 24 compared with placebo and each monotherapy component (see Table 1). LAVENTAIR ELLIPTA showed a statistically significant reduction in SGRQ total score compared with tiotropium in one of the three active- comparator studies (see Table 1).
The proportion of patients who responded with at least the MCID in SGRQ score (defined as a decrease of 4 units from baseline) at Week 24 was greater for LAVENTAIR ELLIPTA (49%) compared with placebo (34%) and each monotherapy component (44% for umeclidinium and 48% for vilanterol). In one active-comparator study, a higher percentage of patients receiving LAVENTAIR ELLIPTA responded with a clinically meaningful improvement in SGRQ score at Week 24 (53%) compared to tiotropium (46%). In the other two active-comparator studies, a similar proportion of patients achieved at least the MCID with LAVENTAIR ELLIPTA and tiotropium; 49% and 54% for LAVENTAIR ELLIPTA 55/22 micrograms and 52% and 55% for tiotropium.
Use of rescue medication
LAVENTAIR ELLIPTA reduced the use of rescue medication with salbutamol over Weeks 1-24 compared with placebo and umeclidinium (see Table 1) and demonstrated an increase from baseline in the proportion of days when no rescue medication was needed (on average 11.1%) compared with a decrease from baseline on placebo (on average 0.9%).
In the three 6-month active-comparator-controlled studies, LAVENTAIR ELLIPTA reduced the use of rescue medication with salbutamol compared with tiotropium, with statistically significant reductions observed in two of the studies (see Table 1). LAVENTAIR ELLIPTA also demonstrated a greater increase from baseline in the proportion of days when no rescue medication was needed in all three studies (average within the range 17.6% to 21.5%) compared with tiotropium (average within the range 11.7% to 13.4%).
Table 1. Lung function, symptomatic and health related quality of life outcomes at Week 24:
A higher dose of umeclidinium/vilanterol (113/22 micrograms) was also studied in a 24-week placebo controlled clinical study and in two of the three 24-week active-controlled studies. The results were similar to those for the LAVENTAIR ELLIPTA dose and provided additional supportive evidence for the efficacy of LAVENTAIR ELLIPTA.
COPD exacerbations
In a 24-week placebo-controlled study in patients with symptomatic COPD, LAVENTAIR ELLIPTA reduced the risk of a moderate/severe COPD exacerbation by 50% compared with placebo (based on analysis of time to first exacerbation: Hazard Ratio (HR) 0.5; 95% CI: 0.3, 0.8; p=0.004*); by 20% compared with umeclidinium (HR 0.8; 95% CI: 0.5, 1.3; p=0.391); and by 30% compared with vilanterol (HR 0.7; 95% CI: 0.4, 1.1; p=0.121). From the three active-comparator studies in patients with symptomatic COPD, the risk of a moderate/severe COPD exacerbation compared with tiotropium was reduced by 50% in one study (HR 0.5; 95% CI: 0.3, 1.0; p=0.044). In the other two studies, the risk of a moderate/severe COPD exacerbation was increased by 20% and 90% (HR 1.2; 95% CI: 0.5, 2.6; p=0.709 and HR 1.9; 95% CI: 1.0, 3.6; p=0.062 respectively). These studies were not specifically designed to evaluate the effect of treatments on COPD exacerbations and patients were withdrawn from the study if an exacerbation occurred.
Supporting efficacy studies
In a randomised, double-blind, 52-week study (CTT116855, IMPACT), 10,355 adult patients with symptomatic COPD and a history of 1 or more moderate/severe exacerbations in the prior 12 months were randomised (1:2:2) to receive umeclidinium/vilanterol (UMEC/VI 55/22 micrograms), fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI 92/55/22 micrograms), or fluticasone furoate/vilanterol (FF/VI 92/22 micrograms) administered once daily as a single inhaler. The primary endpoint was annual rate of on-treatment moderate and severe exacerbations in subjects treated with FF/UMEC/VI compared with FF/VI and UMEC/VI. The mean annual rate of exacerbations was 0.91, 1.07 and 1.21 for FF/UMEC/VI, FF/VI, and UMEC/VI respectively.
The comparison of FF/UMEC/VI to FF/VI and UMEC/VI resulted in a statistically significant 14.8% reduction in risk of a moderate/severe exacerbation (based on analysis of time to first exacerbation) (Hazard Ratio 0.85; 95% CI: 0.80, 0.91; p<0.001) and 16.0% reduction in risk of a moderate/severe exacerbation respectively (based on analysis of time to first exacerbation) (Hazard Ratio 0.84; 95% CI: 0.78, 0.91; p<0.001).
Exercise endurance and lung volumes
LAVENTAIR ELLIPTA 55/22 micrograms improved exercise endurance time compared with placebo, as evaluated with the endurance shuttle walk test (ESWT), in one study but not the second and improved lung volume measures compared with placebo in both studies in adult COPD patients with hyperinflation (functional residual capacity [FRC] >120%). In the first study, LAVENTAIR ELLIPTA 55/22 micrograms demonstrated a statistically significant and clinically relevant improvement (based on a minimal clinically important difference (MCID) between 45 to 85 seconds) over placebo in exercise endurance time (EET) obtained 3 hours after dosing at Week 12 (69.4 seconds [p=0.003]). Improvement in EET compared with placebo was seen at Day 2 and was sustained at Week 6 and Week 12. In the second study, the treatment difference in EET between LAVENTAIR ELLIPTA 55/22 micrograms and placebo was 21.9 seconds (p=0.234) at Week 12.
LAVENTAIR ELLIPTA 55/22 micrograms also showed statistically significant improvements compared with placebo in change from baseline in lung volume measures at trough and at 3 hours post dose at Week 12 in the first study (inspiratory capacity: 237 ml and 316 ml respectively, residual volume: -466 ml and -643 ml respectively and functional residual capacity: -351 ml and -522 ml respectively; all p<0.001). In the second study, LAVENTAIR ELLIPTA 55/22 micrograms showed improvements compared with placebo in change from baseline in lung volume measures at trough and at 3 hours post dose at Week 12 (inspiratory capacity: 198 ml and 238 ml respectively, residual volume: -295 ml and -351 ml respectively and functional residual capacity: -238 ml and -302 ml respectively); all p<0.001*).
* A step-down statistical testing procedure was used in this study and this comparison was below a comparison that did not achieve statistical significance. Therefore, statistical significance on this comparison cannot be inferred.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with LAVENTAIR ELLIPTA in all subsets of the paediatric population in COPD (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
When umeclidinium and vilanterol were administered in combination by the inhaled route, the pharmacokinetics of each component was similar to those observed when each active substance was administered separately. For pharmacokinetic purposes each component can therefore be considered separately.
Absorption
Umeclidinium
Following inhaled administration of umeclidinium in healthy volunteers, Cmax occurred at 5 to 15 minutes. The absolute bioavailability of inhaled umeclidinium was on average 13% of the dose, with negligible contribution from oral absorption. Following repeat dosing of inhaled umeclidinium, steady state was achieved within 7 to 10 days with 1.5 to 1.8-fold accumulation.
Vilanterol
Following inhaled administration of vilanterol in healthy volunteers, Cmax occurred at 5 to 15 minutes. The absolute bioavailability of inhaled vilanterol was 27%, with negligible contribution from oral absorption. Following repeat dosing of inhaled vilanterol, steady state was achieved within 6 days with up to 2.4-fold accumulation.
Distribution
Umeclidinium
Following intravenous administration to healthy volunteers, the mean volume of distribution was 86 litres. In vitro plasma protein binding in human plasma was on average 89%.
Vilanterol
Following intravenous administration to healthy volunteers, the mean volume of distribution at steady state was 165 litres. In vitro plasma protein binding in human plasma was on average 94%.
Biotransformation
Umeclidinium
In vitro studies showed that umeclidinium is primarily metabolised by cytochrome P450 2D6 (CYP2D6) and is a substrate for the P-glycoprotein (P-gp) transporter. The primary metabolic routes for umeclidinium are oxidative (hydroxylation, O-dealkylation) followed by conjugation (glucuronidation, etc), resulting in a range of metabolites with either reduced pharmacological activity or for which the pharmacological activity has not been established. Systemic exposure to the metabolites is low.
Vilanterol
In vitro studies showed that vilanterol is primarily metabolised by cytochrome P450 3A4 (CYP3A4) and is a substrate for the P-gp transporter. The primary metabolic routes for vilanterol are O-dealkylation to a range of metabolites with significantly reduced beta1- and beta2-adrenergic agonist activity. Plasma metabolic profiles following oral administration of vilanterol in a human radiolabel study were consistent with high first-pass metabolism. Systemic exposure to the metabolites is low.
Elimination
Umeclidinium
Plasma clearance following intravenous administration was 151 litres/hour. Following intravenous administration, approximately 58% of the administered radiolabelled dose (or 73% of the recovered radioactivity) was excreted in faeces by 192 hours post-dose. Urinary elimination accounted for 22% of the administered radiolabelled dose by 168 hours (27% of recovered radioactivity). The excretion of the drug-related material in the faeces following intravenous dosing indicated secretion into the bile. Following oral administration to healthy male volunteers, total radioactivity was excreted primarily in faeces (92% of the administered radiolabelled dose or 99% of the recovered radioactivity) by 168 hours post-dose. Less than 1% of the orally administered dose (1% of recovered radioactivity) was excreted in urine, suggesting negligible absorption following oral administration. Umeclidinium plasma elimination half-life following inhaled dosing for 10 days averaged 19 hours in healthy volunteers, with 3% to 4% excreted unchanged in urine at steady-state.
Vilanterol
Plasma clearance of vilanterol following intravenous administration was 108 litres/hour. Following oral administration of radiolabelled vilanterol, mass balance showed 70% of the radiolabel in urine and 30% in faeces. Primary elimination of vilanterol was by metabolism followed by excretion of metabolites in urine and faeces. Vilanterol plasma elimination half-life following inhaled dosing for 10 days averaged 11 hours.
Characteristics in specific groups of healthy volunteers or patients
Elderly
A population pharmacokinetic analysis showed that pharmacokinetics of umeclidinium and vilanterol were similar between COPD patients 65 years and older and those younger than 65 years of age.
Renal impairment
Patients with severe renal impairment showed no evidence of an increase in systemic exposure to either umeclidinium or vilanterol (Cmax and AUC) following administration of umeclidinium/vilanterol with umeclidinium at twice the recommended dose and vilanterol at the recommended dose and no evidence of altered protein binding between patients with severe renal impairment and healthy volunteers.
Hepatic impairment
Patients with moderate hepatic impairment (Child-Pugh Class B) showed no evidence of an increase in systemic exposure to either umeclidinium or vilanterol (Cmax and AUC) following administration of umeclidinium/vilanterol with umeclidinium at twice the recommended dose and vilanterol at the recommended dose and no evidence of altered protein binding between patients with moderate hepatic impairment and healthy volunteers. Umeclidinium/vilanterol has not been evaluated in patients with severe hepatic impairment.
Other special populations
A population pharmacokinetic analysis showed that no dose adjustment is required for umeclidinium or vilanterol based on the effect of age, race, gender, inhaled corticosteroid use, or weight. A study in CYP2D6 poor metabolisers showed no evidence of a clinically significant effect of CYP2D6 genetic polymorphism on systemic exposure to umeclidinium.
Preclinical safety data
In nonclinical studies with umeclidinium and vilanterol, alone and in combination, findings were those typically associated with the primary pharmacology of either muscarinic receptor antagonists or beta2-adrenergic agonists respectively and/or local irritancy. The following statements reflect studies conducted on the individual components.
Genotoxicity and carcinogenicity
Umeclidinium was not genotoxic in a standard battery of studies and was not carcinogenic in lifetime inhalation studies in mice or rats at exposures ≥26 or ≥22-fold, times the human clinical exposure of umeclidinium 55 micrograms, based on AUC, respectively.
In genetic toxicity studies, vilanterol (as alpha-phenylcinnamate) and triphenylacetic acid were not genotoxic indicating that vilanterol (as trifenatate) does not represent a genotoxic hazard to humans. Consistent with findings for other beta2adrenergic agonists, in lifetime inhalation studies, vilanterol trifenatate caused proliferative effects in the female rat and mouse reproductive tract and in the rat pituitary gland. There was no increase in tumour incidence in rats or mice at exposures 0.5 or 13-fold, times the human clinical exposure of vilanterol 22 micrograms based on AUC, respectively.
Toxicity to reproduction
Umeclidinium was not teratogenic in rats or rabbits. In a pre- and post-natal study, subcutaneous administration of umeclidinium to rats resulted in lower maternal body weight gain and food consumption and slightly decreased pre-weaning pup body weights in dams given 180 micrograms/kg/day dose (approximately 80-times the human clinical exposure of umeclidinium 55 micrograms, based on AUC).
Vilanterol was not teratogenic in rats. In inhalation studies in rabbits, vilanterol caused effects similar to those seen with other beta2-adrenergic agonists (cleft palate, open eyelids, sternebral fusion and limb flexure/malrotation) at 6-times the human clinical exposure based on AUC. When given subcutaneously there were no effects at 36-times the human clinical exposure of vilanterol 22 micrograms, based on AUC.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.