LECTRUM Powder for suspension for injection Ref.[50746] Active ingredients: Leuprorelin
Source: Marketing Authorisation Holder Revision Year: 2019
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Endocrine therapy, Hormones and related agents, Gonadotropin releasing hormone analogues
ATC code: L02AE02
Leuprolide acetate, the active substance of the medicinal product Lectrum, is a synthetic nonapeptide analogue of the naturally occurring gonadotrophin releasing hormone (GnRH or LHRH). It has greater potency than the natural hormone, acts as an inhibitor of gonadotrophin production and is chemically distinct from the steroids. Lectrum is provided as a sterile lyophilised powder which, when added to the diluent, becomes a suspension for intramuscular use. Leuprolide acetate is inactive when administered orally.
Lectrum, an LHRH agonist, acts as a powerful gonadotrophin secretion inhibitor, when given continuously and at therapeutic doses. Studies have shown that following initial gonadotrophin stimulation, the chronic administration of Lectrum results in the stopping of ovarian and testicular steroidogenesis. This effect is reversible with discontinuation of therapy. In human subjects, the administration of Lectrum results in an initial increase in circulating levels of the luteinising hormone (LH) and the follicle-stimulating hormone (FSH), leading to a transient increase in gonadal steroid levels (testosterone and dihydrotestosterone in men; and oestrone and oestradiol in premenopausal women).
However, the continuous administration of Lectrum in the recommended doses results in decreased LH, FSH and sex steroid levels. In men, testosterone is reduced to castrate levels. In premenopausal women, oestrogens are reduced to post-menopausal levels. This hormone level reduction takes place within one month after the start of treatment.
Prostate cancer
Prostate growth and function are dependent on the male hormone, testosterone. Achieving a state of androgenic deprivation is the primary objective of the treatment of advanced prostate cancer. In men, the continuous administration of Lectrum results in the decrease of testosterone to prepubertal levels or similar to those obtained with surgical castration.
In patients with metastatic castration resistant prostate cancer, clinical studies have shown benefit from the addition of secondary agents to treatment with LHRH agonists such as Lectrum. Androgen deprivation therapy (ADT) is generally continued in conjunction with secondary therapies after progression on the initial ADT regimen.
Gynaecological use
Since oestrogen stimulates uterine or endometrial tissue growth, medical treatment with Lectrum for uterine fibroids and endometriosis is based on stopping oestrogen production.
Uterine fibroid
The uterine leiomyoma (uterine fibroma) is a gynaecological disorder characterized by the presence of benign tumours of myometrial origin, the growth of which is promoted by oestrogen. The hypo-oestrogenic state resulting from the administration of Lectrum 3.75 mg reduces fibromas and decreases uterine size and volume, eliminating or alleviating symptoms of pelvic pain, menorrhagia, pressure and discomfort. Improvement of haematocrit and haemoglobin levels was observed after the reduction and elimination of menorrhagia.
Anaemia resulting from uterine fibroid, endometriosis and breast cancer
The initial stimulation of gonadotrophins by the anterior pituitary is followed by prolonged suppression. The release of gonadotrophin by the anterior pituitary temporarily increases oestrone and oestradiol levels in premenopausal women. However, the continuous administration of Lectrum causes a reduction in oestradiol, oestrone and progesterone concentrations to postmenopausal levels. As a consequence of suppressed ovarian function, both normal endometrial as well as ectopic tissues become inactive and atrophic, resulting in amenorrhoea in women.
Endometriosis
The etiology of endometriosis is unclear, although there are several theories. The most likely cause is retrograde menstruation, but other possible origins include surgical transplantation and direct endometrial extension. The hypo-oestrogenic state resulting from the administration of Lectrum 3.75 mg causes atrophic changes in uterine and endometrial tissues, which allow the process to be resolved and which include the reduction of endometrial implants, an impediment to new lesions and a possible reduction in adhesions: this will result in the reduction of pain and other symptoms. The suppression of pituitary gonadotrophins frequently results in elimination of the menstrual cycle. After discontinuing a cycle of treatment with Lectrum 3.75 mg for six months, the average return time of menstruation was 52 days (range: 7-183 days). Because oestrogenic and androgenic steroidogenesis is suppressed, the androgenic effects present with other therapies are avoided.
Precocious puberty
Children
Reversible suppression of pituitary gonadotropin release occurs, with a subsequent decrease in oestradiol (E2) or testosterone levels to values in the pre-pubertal range.
Initial gonadal stimulation (flare-up) may cause vaginal bleeding in girls who are already postmenarchal at start of treatment. Withdrawal bleeding may occur at the start of treatment. The bleeding normally stops as treatment continues.
The following therapeutic effects can be demonstrated:
- Suppression of basal and stimulated gonadotropin levels to pre-pubertal levels;
- Suppression of prematurely increased sexual hormone levels to pre-pubertal levels and arrest of premature menstruation;
- Arrest/involution of somatic pubertal development (Tanner stages);
- Improvement/normalisation of the ratio of chronological age to bone age;
- Prevention of progressive bone age acceleration;
- Decrease of growth velocity and its normalisation;
- Increase in final height.
Treatment result is the suppression of the pathologically, prematurely activated hypothalamicpituitary-gonadal axis according to pre-pubertal age.
In a long-term clinical trial in children treated with Lectrum at doses up to 15 mg monthly for >4 years resumption of pubertal progression were observed after cessation of treatment. Follow up of 20 female subjects to adulthood showed normal menstrual cycles in 80% and 12 pregnancies in 7 of the 20 subjects including multiple pregnancies for 4 subjects.
5.2. Pharmacokinetic properties
Absorption
Bioavailability by subcutaneous administration is comparable to that by intravenous administration.
Distribution
The volume of distribution in healthy male volunteers after a single intravenous dose was 27 l. Plasma protein binding is moderate (46%). In humans, metabolism, distribution and excretion of Lectrum are not totally determined. The pharmacokinetics of the drug in patients with hepatic and renal dysfunction have not been determined.
Peak serum concentrations achieved 1 month after a single administration of Lectrum in patients with prostate cancer, at doses of 3.75 mg or 7.5 mg, given subcutaneously or intramuscularly, were 0.7 ng/ml and 1.0 ng/ml.
The peak plasma concentration of Lectrum after an injection of Lectrum 7.5 mg in adult patients was nearly 20 ng/ml in 4 hours, declining to 0.36 ng/ml in 4 weeks. There is no evidence of drug accumulation in the body.
Biotransformation
Lectrum is metabolised to smaller, inactive peptides. Metabolite I is a pentapeptide, metabolites II and III are tripeptides and metabolite IV is a dipeptide. In healthy volunteers, the half-life is approximately 3 hours after the administration of a 1 mg intravenous dose.
Elimination
Less than 5% of a 3.75 dose was recovered in urine as an unmetabolised drug and as a metabolite.
Special populations
The pharmacokinetics of the medicinal product in hepatically and renally impaired patients has not been determined.
Drug interactions
No pharmacokinetic-based drug-drug interaction studies have been conducted with Lectrum. However, because Lectrum acetate is a peptide that is primarily degraded by peptidase and not by cytochrome P-450 enzymes as noted in specific studies, and the medicinal product is only about 46% bound to plasma proteins, drug interactions would not be expected to occur.
Lectrum 3.75 mg and Lectrum 11.25 mg
Precocious puberty
Children
Lectrum 3.75 mg:
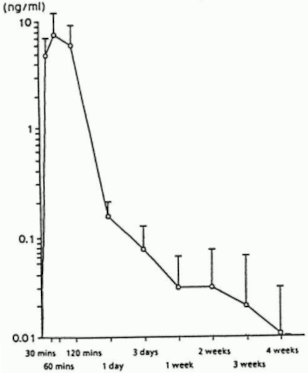
Figure 1 presents Lectrum serum levels after a single s.c. administration of Lectrum depot at a dose of 30 μg/kg body weight. Peak serum levels are reached sixty minutes after administration (7.81 ± 3.59 ng/ml). The AUC0-672 is 105.78 ± 52.40 ng x hr/ml.
Figure 1: Lectrum serum levels after single s.c. administration of 30 μg/kg body weight of Lectrum as depot formulation (n=6) (Mean ±SD)
Lectrum 11.25 mg:
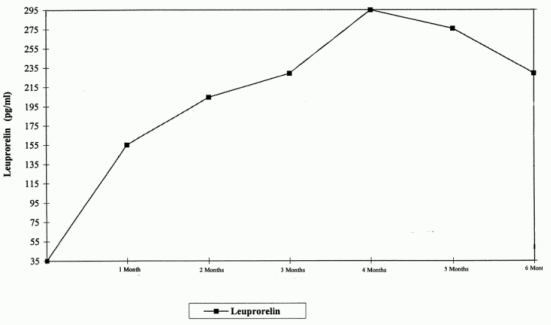
Figure 1 presents the Lectrum serum levels in children during the first 6 months of treatment following sc. administration of Lectrum 3-month depot (two injections).
From the first injection, the Lectrum serum levels increase reaching maximal serum levels at month 4 (294.79 pg/ml ± 105.42) and slightly decrease until month 6 (229.02 pg/ml ± 103.33).
Figure 1: Lectrum serum levels during the first six months of treatment with the Lectrum 3-month depot formulation (two sc. injections) (n=42-43)
5.3. Preclinical safety data
Carcinogenesis, mutagenesis, fertility damage, teratogenesis
Carcinogenicity studies conducted in animals (rats and mice) for a period of two years revealed that in rats there was a dose-related incidence of pituitary hyperplasia and benign pituitary adenomas when the medication was administered subcutaneously for 24 months at high daily doses (0.6 to 4 mg/kg). In women and men, respectively, a significant increase in pancreatic islet adenomas and testicular interstitial cell adenomas was also observed, although this was not dose-related (a greater incidence was found in the low-dose groups). In mice, no pituitary abnormality was observed with doses as high as 60 mg/kg for 2 years. Patients received Lectrum for over 3 years in doses as high as 10 mg/day and for 2 years with doses as high as 20 mg/day. Clinical signs and pituitary abnormalities were not observed in any of these patients. Mutagenicity studies have been carried out with Lectrum in bacterial systems and mammals. Such studies have not revealed evidence of a mutagenic potential for this drug. Clinical and pharmacological studies in adults, with a similar analogue or with Lectrum, have shown a complete reversal of fertility suppression when the medication is withdrawn after continuous administration for periods of up to 24 weeks. Although clinical studies in children to assess the reversibility of fertility suppression have not been completed, animal studies with Lectrum and other analogues have shown functional recovery. However, a study of Lectrum in immature male rats demonstrated testicular tubular degeneration, even after the recovery period. In spite of the failure/shortcoming in histological recovery, the treated animals proved to be as fertile as the controls. No histological changes were observed in female rats.
The offspring of treated animals of both sexes were normal. The effect of treatment of the parents on the reproductive development of the F1 generation has not been studied. The clinical significance of all these findings is unknown. When administered on the sixth day of pregnancy at test dosages of 0.00024, 0.0024 and 0.024 mg/kg (1/600 to 1/6 of the human dose) in rabbits, Lectrum caused a dose-related increase in fetal malformations. There was an increase in fetal mortality and a decrease in fetal weight with the use of the two higher doses in rabbits and the highest dose in rats. The effects on fetal mortality are the logical result of changes in hormone levels caused by this medication.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.