LEVECETAM Film-coated tablet Ref.[50751] Active ingredients: Levetiracetam
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2022 Publisher: Dr. Reddys Laboratories (Australia) Pty Ltd, Suite 3.03, Level 3, 390 St Kilda Road, MELBOURNE, VIC, AUSTRALIA Phone: 1800 733 397
Product name and form
Levetiracetam.
| Pharmaceutical Form |
|---|
|
Levecetam 250 levetiracetam 250 mg are blue coloured, oval shaped, film-coated tablets debossed with breakline separating ‘250’ and ‘MG’ on one side and ‘1014’ on other side. Levecetam 500 levetiracetam 500 mg are yellow coloured, oval shaped, film-coated tablets debossed with breakline separating ‘500’ and ‘MG’ on one side and ‘1015’ on other side. Levecetam 1000 levetiracetam 1000 mg are white to off white, oval shaped, film-coated tablets debossed with breakline separating ‘1000’ and ‘MG’ on one side and ‘1017’ on other side. |
Qualitative and quantitative composition
The active ingredient in Levecetam 250, 500, 1000/tablets is levetiracetam 250 mg, 500 mg or 1000 mg.
For the full list of excipients, see Section 6.1 List of excipients.
Physicochemical properties
Levetiracetam is a pure enantiomer. It is a white to off white powder with a faint odour and a bitter taste. It is very soluble in water (104 g/100 mL). It is freely soluble in chloroform (65.3 mg/100 mL) and in methanol (53.6 g/100mL), soluble in ethanol (16.5 g/100 mL), sparingly soluble in acetonitrile (5.7 g/100 mL) and practically insoluble in n-hexane.
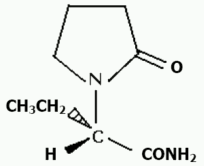
Chemical name: (S)-a-ethyl-2-oxo-1-pyrrolidineacetamide.
Chemical structure:
CAS number: 102767-28-2
Molecular Formula: C8H14N2O2.
Molecular weight: 170.2
| Active Ingredient |
|---|
|
Levetiracetam, is a pyrrolidone derivative, chemically unrelated to existing antiepileptic active substances. Levetiracetam induces seizure protection in a broad range of animal models of partial and primary generalised seizures without having a pro-convulsant effect. The primary metabolite is inactive. |
| List of Excipients |
|---|
|
All strengths of tablet also contains the following excipients: maize starch, povidone, purified talc, colloidal anhydrous silica, sodium starch glycollate type A, silicon dioxide and magnesium stearate. 250 mg tablet coating: hypromellose, titanium dioxide, macrogol 400, indigo carmine CI73015. 500 mg tablet coating: hypromellose, titanium dioxide, macrogol 400 and iron oxide yellow CI77492. 1000 mg tablet coating: hypromellose, macrogol 400 and titanium dioxide |
Pack sizes and marketing
Levecetam 250, 500, 1000 tablets come in 3 different strengths in packs of 60 tablets packed in PVC/PVDC/Al of blister pack.
Marketing authorization holder
Dr. Reddy’s Laboratories (Australia) Pty Ltd, Suite 3.03, Level 3, 390 St Kilda Road, MELBOURNE, VIC, AUSTRALIA
Phone: 1800 733 397
Marketing authorization dates and numbers
Date of first approval: 29 April 2010
Drugs
| Drug | Countries | |
|---|---|---|
| LEVECETAM | Australia, Ecuador |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.