LIPTRUZET Tablet Ref.[51015] Active ingredients: Atorvastatin Ezetimibe
Source: FDA, National Drug Code (US) Revision Year: 2014
Product description
LIPTRUZET contains ezetimibe, a selective inhibitor of intestinal cholesterol and related phytosterol absorption, and atorvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor.
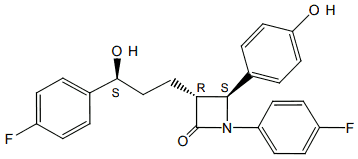
The chemical name of ezetimibe is 1-(4-fluorophenyl)3==®==[3-(4-fluorophenyl)-3(S)hydroxypropyl] 4(S)(4-hydroxyphenyl)-2-azetidinone. The empirical formula is C24H21F2NO3. Its molecular weight is 409.4.
Ezetimibe is a white, crystalline powder that is freely to very soluble in ethanol, methanol, and acetone and practically insoluble in water. Its structural formula is:
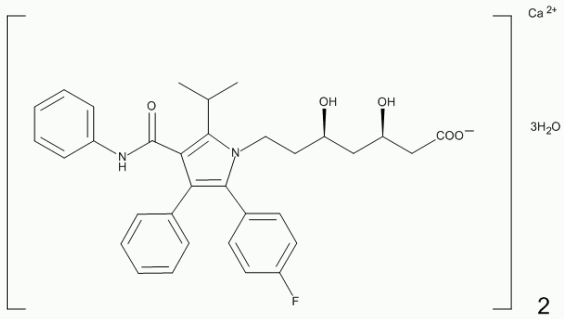
Atorvastatin is [R-(R*, R*)]2(4-fluorophenyl)-β, δ,-dihydroxy-5-(1-methylethyl)-3-phenyl-4 [(phenylamino) carbonyl]-1H-pyrrole-1-heptanoic acid, calcium salt (2:1) trihydrate.
Atorvastatin calcium is a white or almost white crystalline powder that is soluble in dimethyl sulfoxide.
The degree of solubility in water, ethanol, and methylene chloride is very slightly soluble to practically insoluble. The molecular formula of atorvastatin calcium is C66H68CaF2N4O10.3H2O. The molecular weight of atorvastatin calcium is 1209.36.
Its structural formula is:
LIPTRUZET is available for oral use as tablets containing 10 mg of ezetimibe and: 10.9 mg of atorvastatin calcium, equivalent to 10 mg of atorvastatin (LIPTRUZET 10 mg/10 mg); 21.7 mg of atorvastatin calcium, equivalent to 20 mg of atorvastatin (LIPTRUZET 10 mg/20 mg); 43.4 mg of atorvastatin calcium, equivalent to 40 mg of atorvastatin (LIPTRUZET 10 mg/40 mg); or 86.8 mg of atorvastatin calcium, equivalent to 80 mg of atorvastatin (LIPTRUZET 10 mg/80 mg). Each film-coated tablet of LIPTRUZET contains the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, povidone, sodium lauryl sulfate, magnesium stearate, hydroxypropyl cellulose, calcium carbonate, colloidal silicon dioxide, and polysorbate. In addition, the film coating contains the following inactive ingredients: hydroxypropyl methylcellulose/hypromellose, macrogol/polyethylene glycol, titanium dioxide, and talc.
| Dosage Forms and Strengths |
|---|
|
| How Supplied |
|---|
|
Tablets LIPTRUZET 10 mg/10 mg are white to off-white capsule-shaped, biconvex film-coated tablets with code “257” on one side. They are supplied as follows: NDC 0006-0257-02 unit of use packages of 30 (one carton containing one multi-fold wallet with two 15-count blister cards). NDC 0006-0257-03 unit of use packages of 90 (three cartons each containing one multi-fold wallet with two 15-count blister cards). Tablets LIPTRUZET 10 mg/20 mg are white to off-white capsule-shaped, biconvex film-coated tablets with code “333” on one side. They are supplied as follows: NDC 0006-0333-02 unit of use packages of 30 (one carton containing one multi-fold wallet with two 15-count blister cards). NDC 0006-0333-03 unit of use packages of 90 (three cartons each containing one multi-fold wallet with two 15-count blister cards). Tablets LIPTRUZET 10 mg/40 mg are white to off-white capsule-shaped, biconvex film-coated tablets with code “337” on one side. They are supplied as follows: NDC 0006-0337-02 unit of use packages of 30 (one carton containing one multi-fold wallet with two 15-count blister cards). NDC 0006-0337-03 unit of use packages of 90 (three cartons each containing one multi-fold wallet with two 15-count blister cards). Tablets LIPTRUZET 10 mg/80 mg are white to off-white capsule-shaped, biconvex film-coated tablets with code “357” on one side. They are supplied as follows: NDC 0006-0357-02 unit of use packages of 30 (one carton containing one multi-fold wallet with two 15-count blister cards). NDC 0006-0357-03 unit of use packages of 90 (three cartons each containing one multi-fold wallet with two 15-count blister cards). 3 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ 08889, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| LIPTRUZET | Cyprus, Germany, France |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.