LITHOSTAT Tablet Ref.[50673] Active ingredients: Acetohydroxamic acid
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
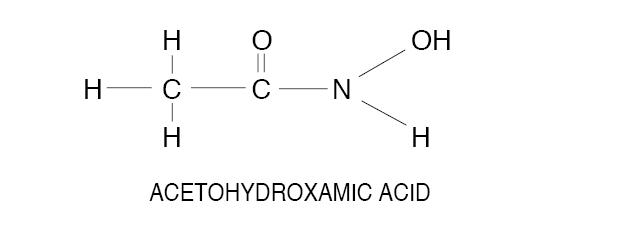
Acetohydroxamic acid (AHA) is a stable, synthetic compound derived from hydroxylamine and ethyl acetate. Its molecular structure is similar to urea:
AHA is weakly acidic, highly soluble in water, and chelates metals - notably iron. The molecular weight is 75.068. AHA has a pKa of 9.32 and a melting point of 89-91° C. AHA is a urease inhibitor. Available as 250 mg tablets.
| How Supplied |
|---|
|
LITHOSTAT, NDC 0178-0500-01, is available for oral administration as 250 mg white, round tablets, in unit of use packages of 100 tablets. Each LITHOSTAT tablet is debossed MPC 500 on one side and blank on the other side. MISSION PHARMACAL COMPANY, San Antonio, TX 78230 1355 |
Drugs
| Drug | Countries | |
|---|---|---|
| LITHOSTAT | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.