LOPRESSOR Tablet Ref.[28037] Active ingredients: Metoprolol
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
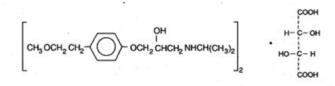
Lopressor, metoprolol tartrate USP is a selective beta1-adrenoreceptor blocking agent, available as 50 mg and 100 mg tablets for oral administration. Metoprolol tartrate USP is (±)-1-(Isopropylamino)-3-[p-(2-methoxyethyl)phenoxy]-2-propanol L-(+)-tartrate (2:1) salt, and its structural formula is
Metoprolol tartrate USP is a white, practically odorless, crystalline powder with a molecular weight of 684.82. It is very soluble in water; freely soluble in methylene chloride, in chloroform, and in alcohol; slightly soluble in acetone; and insoluble in ether.
Inactive Ingredients: Tablets contain cellulose compounds, colloidal silicon dioxide, D&C Red No. 30 aluminum lake (50 mg tablets), FD&C Blue No. 2 aluminum lake (100 mg tablets), lactose, magnesium stearate, polyethylene glycol, propylene glycol, povidone, sodium starch glycolate, talc, and titanium dioxide.
| How Supplied |
|---|
Lopressor Tabletsmetoprolol tartrate USP Tablets Tablets 50 mg: capsule-shaped, biconvex, pink, scored (imprinted LOPRESSOR on one side and 458 twice on the scored side) Bottles of 100 NDC 30698-458-01 Tablets 100 mg: capsule-shaped, biconvex, light blue, scored (imprinted LOPRESSOR on one side and 459 twice on the scored side) Bottles of 100 NDC 30698-459-01 Manufactured for and Distributed by: Validus Pharmaceuticals LLC, 119 Cherry Hill Road, Suite 310, Parsippany, NJ 07054 |
Drugs
| Drug | Countries | |
|---|---|---|
| LOPRESSOR | Brazil, France, Tunisia, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.