MACUGEN Solution for injection Ref.[9039] Active ingredients: Pegaptanib
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: PharmaSwiss Česká republika s.r.o., Jankovcova 1569/2c, 170 00 Praha 7, Czech Republic
Pharmacodynamic properties
Pharmacotherapeutic group: Ophthalmologicals, Ocular vascular disorder agents
ATC Code: S01LA03
Mechanism of action
Pegaptanib is a pegylated modified oligonucleotide that binds with high specificity and affinity to extracellular Vascular Endothelial Growth Factor (VEGF165) inhibiting its activity. VEGF is a secreted protein that induces angiogenesis, vascular permeability and inflammation, all of which are thought to contribute to the progression of the neovascular (wet) form of AMD.
Pharmacodynamic effects
VEGF165 is the VEGF isoform preferentially involved in pathological ocular neovascularisation. The selective inhibition in animals with pegaptanib proved as effective at suppressing pathological neovascularisation as pan-VEGF inhibition, however pegaptanib spared the normal vasculature whereas pan-VEGF inhibition did not.
Reductions in the growth of mean total lesion size, choroidal neovascularisation (CNV size), and fluorescein leak size, have been shown in patients with AMD treated with Macugen.
Clinical efficacy and safety
Pegaptanib was studied in two controlled, double-masked, and identically designed randomised studies (EOP1003; EOP1004) in patients with neovascular AMD. A total of 1190 patients were treated (892 pegaptanib, 298 sham (control)) with a median age of 77 years. Patients received a mean of between 8.4-8.6 treatments out of possible 9 total across all treatment arms in the first year.
Patients were randomised to receive sham or 0.3 mg, 1 mg or 3 mg pegaptanib administered as intravitreal injections every 6 weeks for 48 weeks. Verteporfin photodynamic therapy (PDT) was permitted in patients with predominantly classic lesions at the discretion of the investigators.
The two trials enrolled patients, including all neovascular AMD lesion subtypes (25% predominantly classic, 39% occult with no classic and 36% minimally classic), lesion sizes up to 12 disc areas, of which up to 50% could be comprised of subretinal haemorrhage and/or up to 25% fibrotic scar or atrophic damage. Patients had up to one prior PDT and baseline visual acuity in the study eye between 20/40 and 20/320.
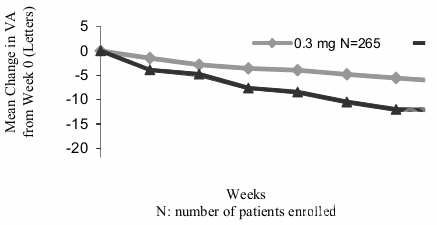
At one year, pegaptanib 0.3 mg exhibited a statistically significant treatment benefit for the primary efficacy endpoint; proportion of patients losing less than 15 letters of visual acuity (prespecified pooled analysis, pegaptanib 0.3 mg 70% versus Sham 55%, p=0.0001; EOP1003 pegaptanib 0.3 mg 73% versus Sham 59%, p=0.0105; EOP1004 pegaptanib 0.3 mg 67% versus Sham 52%, p=0.0031).
Mean Change in Visual Acuity Over Time; Year 1; ITT (LOCF):
Pegaptanib 0.3 mg showed treatment benefit regardless of baseline lesion subtype, lesion size and visual acuity as well as age, gender, iris pigmentation and prior and/or baseline PDT usage.
At the end of the first year (week 54), 1053 patients were re-randomized to either continue or discontinue treatment through week 102.
On average, the treatment benefit was maintained at 102 weeks with continuing preservation of visual acuity for patients re-randomized to continue pegaptanib. Patients who were re-randomized to discontinue pegaptanib after one year, lost visual acuity during the second year.
Summary of Mean Changes in Visual Acuity from Baseline to Weeks 6, 12, 54 and 102 (LOCF):
| EOP 1003 | EOP 1004 | |||||
|---|---|---|---|---|---|---|
| 0,3-0,3 | 0,3-discontinued | Sham-Sham / Sham + discontinued | 0,3-0,3 | 0,3-discontinued | Sham-Sham / Sham + discontinued | |
| N | 67 | 66 | 54 | 66 | 66 | 53 |
| Mean change in VA Week 6 | -1.9 | -0.0 | -4.4 | -1.9 | -2.0 | -3.4 |
| Mean change in VA Week 12 | -4.3 | -2.0 | -4.8 | -2.8 | -2.2 | -4.7 |
| Mean change in VA Week 54 | -9.6 | -4.3 | -11.7 | -8.0 | -7.6 | -15.6 |
| Mean change in VA Week 102 | -10.8 | -9.7 | -13.1 | -8.0 | -12.7 | -21.1 |
Data over a two-year period indicate that Macugen treatment should be initiated as early as possible. In advanced disease the initiation and continuation of Macugen therapy should consider the potential for useful vision in the eye.
Macugen therapy administered to both eyes concurrently has not been studied.
The safety and efficacy of Macugen beyond two years has not been demonstrated.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Macugen in all subsets of the paediatric population in age-related macular degeneration. See section 4.2 for information on paediatric use.
Pharmacokinetic properties
Absorption
In animals, pegaptanib is slowly absorbed into the systemic circulation from the eye after intravitreal administration. The rate of absorption from the eye is the rate-limiting step in the disposition of pegaptanib in animals and is likely to be in humans. In humans, the average ± standard deviation apparent plasma half-life of pegaptanib after a 3 mg (10-times the recommended dose) monocular dose is 10 ± 4 days.
A mean maximum plasma concentration of about 80 ng/ml occurs within 1 to 4 days after a 3 mg monocular dose in humans. The mean area under the plasma concentration-time curve (AUC) is about 25 μg·hr/ml at this dose. Pegaptanib does not accumulate in the plasma when administered intravitreally every 6 weeks. At doses below 0.5 mg/eye, pegaptanib plasma concentrations do not likely exceed 10 ng/ml.
The absolute bioavailability of pegaptanib after intravitreal administration has not been assessed in humans, but is approximately 70-100% in rabbits, dogs and monkeys.
In animals that received doses of pegaptanib up to 0.5 mg/eye to both eyes, plasma concentrations were 0.03% to 0.15% of those in the vitreous humour.
Distribution, biotransformation and elimination
In mice, rats, rabbits, dogs and monkeys, pegaptanib distributes primarily into plasma volume and is not extensively distributed to peripheral tissues after intravenous administration. Twenty-four hours after intravitreous administration of a radiolabeled dose of pegaptanib to both eyes of rabbits, radioactivity was mainly distributed in vitreous humour, retina and aqueous humour. After intravitreal and intravenous administrations of radiolabeled pegaptanib to rabbits, the highest concentrations of radioactivity (excluding the eye for the intravitreal dose) were obtained in the kidney. In rabbits, the component nucleotide, 2'fluorouridine is found in plasma and urine after single radiolabeled pegaptanib intravenous and intravitreal doses. Pegaptanib is metabolised by endo and exonucleases. In rabbits, pegaptanib is eliminated as parent drug and metabolites primarily in the urine.
Special populations
Pegaptanib pharmacokinetics is similar in female and male patients and within the age range 50 to 90 years.
Pegaptanib sodium has not been adequately studied in patients with creatinine clearance below 20 ml/min. A decrease in creatinine clearance down to 20 ml/min may be associated with up to a 2.3-fold increase in pegaptanib AUC. No special considerations are needed in patients with creatinine clearance above 20 ml/min who are treated with the recommended dose of pegaptanib sodium 0.3 mg.
Pegaptanib pharmacokinetics have not been studied in patients with hepatic impairment. The systemic exposure is expected to be within a well tolerated range in patients with hepatic impairment, as a 10 fold higher dose (3 mg/eye) was well tolerated.
Preclinical safety data
Non-clinical data revealed no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity and genotoxicity. There are no studies on the carcinogenic potential of pegaptanib.
Pegaptanib produced no maternal toxicity and no evidence of teratogenicity or foetal mortality in mice at intravenous doses of 1 to 40 mg/kg/day. Reduced body weight (5%) and minimal delayed ossification in forepaw phalanges were observed, only at exposure levels based on AUC of over 300 fold greater than that expected in humans. These finding are therefore considered to be of limited clinical relevance. In the 40 mg/kg/day group, pegaptanib concentrations in the amniotic fluid were 0.05% of the maternal plasma levels. There are no reproductive toxicity studies in rabbits. No data are available to evaluate male or female mating or fertility indices.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.