MACUGEN Solution for injection Ref.[9039] Active ingredients: Pegaptanib
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: PharmaSwiss Česká republika s.r.o., Jankovcova 1569/2c, 170 00 Praha 7, Czech Republic
Therapeutic indications
Macugen is indicated for the treatment of neovascular (wet) age-related macular degeneration (AMD) in adults (see section 5.1).
Posology and method of administration
Macugen should only be administered by ophthalmologists experienced in intravitreal injections.
Posology
The patient's medical history for hypersensitivity reactions should be carefully evaluated prior to performing the intravitreal procedure (see section 4.4).
The recommended dose is 0.3 mg pegaptanib, equivalent to 90 microliters, administered once every six weeks (9 injections per year) by intravitreal injection into the affected eye.
Following the injection, transient increases in intraocular pressure were seen in Macugen treated patients. Therefore, the perfusion of the optic nerve head and intraocular pressure should be monitored. Moreover patients should be closely monitored for vitreous haemorrhage and endophthalmitis in the two weeks following the injection. Patients should be instructed to report any symptoms suggestive of these conditions without delay (see section 4.4).
After 2 consecutive injections of Macugen, if a patient does not demonstrate a treatment benefit (loss of less than 15 letters of visual acuity) at the 12-week visit, consideration should be given to stopping or withholding Macugen therapy.
Special populations
Elderly
No special considerations are needed.
Hepatic impairment
Macugen has not been studied in patients with hepatic impairment.
However, no special considerations are needed in this population (see section 5.2).
Renal impairment
Macugen has not been adequately studied in patients with severe renal impairment. Dose adjustments are not recommended in patients with mild or moderate renal impairment (see section 5.2).
Paediatric population
The safety and efficacy of Macugen in children under 18 years has not yet been established. No data are available.
Method of administration
For intravitreal injection use only. Macugen should be inspected visually for particulate matter and discoloration prior to administration (see section 6.6).
The injection procedure should be carried out under aseptic conditions, which includes the use of surgical hand disinfection, sterile gloves, a sterile drape and a sterile eyelid speculum (or equivalent) and the availability of sterile paracentesis (if required). Adequate anaesthesia and a broad-spectrum topical microbicide should be administered prior to the injection.
The pre-filled syringe is supplied with an excess product volume. Injecting the entire volume of the prefilled syringe could result in overdose (see section 4.8 and 4.9). See section 6.6 for instructions to expel the excess volume before injection.
Overdose
Overdose with Macugen has not been reported in clinical trials.
Overdosing with increased injection volume (e.g. when the excess volume in the pre-filled syringe is not expelled before injection) may elevate intraocular pressure (see section 4.8). Treating physician should always expel excess volume of solution according to instructions under the section 6.6. Therefore, in case of overdose, intraocular pressure should be monitored and if deemed necessary by the treating physician, adequate treatment should be initiated.
Shelf life
Shelf life: 3 years.
Special precautions for storage
Store in a refrigerator (2ºC to 8ºC). Do not freeze.
The solution to be injected should reach room temperature (below 25°C) before injecting.
This medicinal product should be discarded if kept at room temperature for more than two weeks. To prevent contamination, the syringe should not be removed from the pouch until the patient has been prepared for injection.
Nature and contents of container
Each pack contains a pouch in a carton containing a 1 ml pre-filled syringe, Type 1 glass, sealed with an elastomeric (brombutyl rubber) plunger stopper and a pre-attached plunger rod, held by a plastic clip. The syringe has a pre-attached polycarbonate plastic luer lock adaptor and the tip is sealed with an elastomeric (bromobutyl/synthetic isoprene) tip cap.
Each pre-filled syringe contains approximately 0.25-0.27 ml of solution.
Each carton contains one pre-filled syringe in a pouch (single dose pack).
The pack is supplied without a needle.
Special precautions for disposal and other handling
Macugen is for single use only. If the solution appears cloudy, particles are observed or if there is evidence of damage to the syringe, or if the plastic clip is missing or not attached to the syringe, that Macugen dose should not be used.
Prior to the administration, the syringe should be removed from the plastic clip and the tip cap removed. A 27 or 30 G x ½ inch needle should be attached to the luer lock adaptor, to allow the administration of the medicinal product (see Figure 1, below).
CAUTION: Since the pre-filled syringe contains more medicinal product volume (250-270 microlitres) than the recommended dose (90 microlitres), a part of the volume contained in the syringe has to be discarded prior to the administration. Follow the instructions below to expel the excess volume before injection.
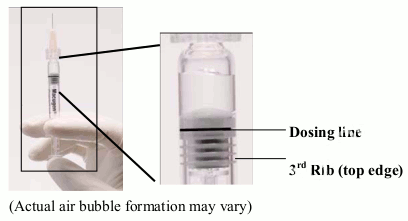
Figure 1. Before expelling air bubble and excess drug:
The syringe should be checked with the needle pointing up for the presence of bubbles. If there are bubbles, the syringe should be gently tapped with a finger until the bubbles rise to the top of the syringe.
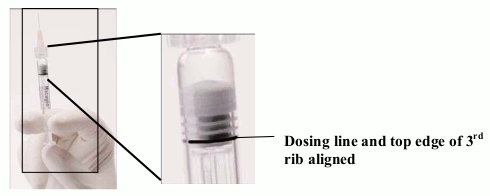
SLOWLY depress the plunger to eliminate all the bubbles and to expel the excess drug so that the top edge of the 3rd rib on the plunger stopper aligns with the pre-printed black dosing line (See Fig 2, below). The plunger stopper should not be pulled back.
Figure 2. After expelling air bubble and excess drug:
At this point, the remaining content of the syringe should be injected.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.