MEKINIST Film-coated tablet Ref.[8867] Active ingredients: Trametinib
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, protein kinase inhibitor, Mitogen-activated protein kinase (MEK) inhibitors
ATC code: L01EE01
Mechanism of action
Trametinib is a reversible, highly selective, allosteric inhibitor of mitogen-activated extracellular signal regulated kinase 1 (MEK1) and MEK2 activation and kinase activity. MEK proteins are components of the extracellular signal-related kinase (ERK) pathway. In melanoma and other cancers, this pathway is often activated by mutated forms of BRAF which activates MEK. Trametinib inhibits activation of MEK by BRAF and inhibits MEK kinase activity. Trametinib inhibits growth of BRAF V600 mutant melanoma cell lines and demonstrates anti-tumour effects in BRAF V600 mutant melanoma animal models.
Combination with dabrafenib
Dabrafenib is an inhibitor of RAF kinases. Oncogenic mutations in BRAF lead to constitutive activation of the RAS/RAF/MEK/ERK pathway. Thus, trametinib and dabrafenib inhibit two kinases in this pathway, MEK and RAF, and therefore the combination provides concomitant inhibition of the pathway. The combination of trametinib with dabrafenib has shown anti-tumour activity in BRAF V600 mutation positive melanoma cell lines in vitro and delays the emergence of resistance in vivo in BRAF V600 mutation positive melanoma xenografts.
Determination of BRAF mutation status
Before taking trametinib or the combination with dabrafenib, patients must have BRAF V600 mutation-positive tumour status confirmed by a validated test.
In clinical trials, central testing for BRAF V600 mutation using a BRAF mutation assay was conducted on the most recent tumour sample available. Primary tumour or tumour from a metastatic site was tested with a validated polymerase chain reaction (PCR) assay developed by Response Genetics Inc. The assay was specifically designed to differentiate between the V600E and V600K mutations. Only patients with BRAF V600E or V600K mutation positive tumours were eligible for study participation.
Subsequently, all patient samples were re-tested using the CE-marked bioMerieux (bMx) THxID BRAF validated assay. The bMx THxID BRAF assay is an allele-specific PCR performed on DNA extracted from FFPE tumour tissue. The assay was designed to detect the BRAF V600E and V600K mutations with high sensitivity (down to 5% V600E and V600K sequence in a background of wild-type sequence using DNA extracted from FFPE tissue). Non-clinical and clinical trials with retrospective bi-directional Sanger sequencing analyses have shown that the test also detects the less common BRAF V600D mutation and V600E/K601E mutation with lower sensitivity. Of the specimens from the non-clinical and clinical trials (n=876) that were mutation positive by the THxID BRAF assay and subsequently were sequenced using the reference method, the specificity of the assay was 94%.
Pharmacodynamic effects
Trametinib suppressed levels of phosphorylated ERK in BRAF mutant melanoma tumour cell lines and melanoma xenograft models.
In patients with BRAF and NRAS mutation positive melanoma, administration of trametinib resulted in dose-dependent changes in tumour biomarkers including inhibition of phosphorylated ERK, inhibition of Ki67 (a marker of cell proliferation), and increases in p27 (a marker of apoptosis). The mean trametinib concentrations observed following repeat dose administration of 2 mg once daily exceeds the preclinical target concentration over the 24-hr dosing interval, thereby providing sustained inhibition of the MEK pathway.
Clinical efficacy and safety
Unresectable or metastatic melanoma
In the clinical trials only patients with cutaneous melanoma were studied. Efficacy in patients with ocular or mucosal melanoma has not been assessed.
Trametinib in combination with dabrafenib
Treatment naïve patients
The efficacy and safety of the recommended dose of trametinib (2 mg once daily) in combination with dabrafenib (150 mg twice daily) for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation was studied in two Phase III studies and one supportive Phase I/II study.
MEK115306 (COMBI-d):
MEK115306 was a Phase III, randomised, double-blinded study comparing the combination of dabrafenib and trametinib to dabrafenib and placebo in first-line therapy for subjects with unresectable (Stage IIIC) or metastatic (Stage IV) BRAF V600E/K mutation-positive cutaneous melanoma. The primary endpoint of the study was progression-free survival (PFS), with a key secondary endpoint of overall survival (OS). Subjects were stratified by lactate dehydrogenase (LDH) level (> the upper limit of normal (ULN) versus ULN) and BRAF mutation (V600E versus V600K).
A total of 423 subjects were randomised 1:1 to either combination (N=211) or dabrafenib (N=212). Most subjects were Caucasian (>99%) and male (53%), with a median age of 56 years (28% were ≥65 years). The majority of subjects had Stage IVM1c disease (67%). Most subjects had LDH ≤ULN (65%), Eastern Cooperative Oncology Group (ECOG) performance status of 0 (72%), and visceral disease (73%) at baseline. The majority of subjects had a BRAF V600E mutation (85%). Subjects with brain metastases were not included in the trial.
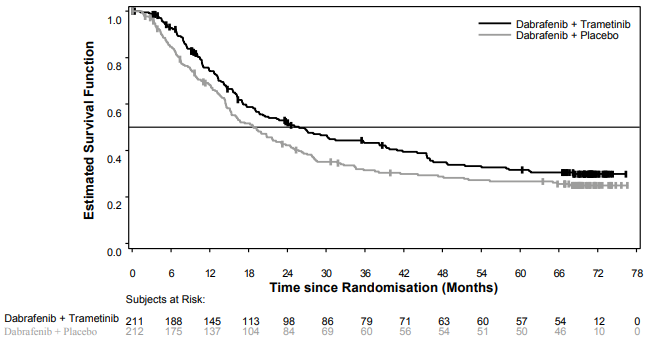
Median OS and estimated 1-year, 2-year, 3-year, 4-year and 5-year survival rates are presented in Table 6. From an OS analysis at 5 years, the median OS for the combination arm was approximately 7 months longer than for dabrafenib monotherapy (25.8 months versus 18.7 months) with 5-year survival rates of 32% for the combination versus 27% for dabrafenib monotherapy (Table 6, Figure 1). The Kaplan-Meier OS curve appears to stabilise from 3 to 5 years (see Figure 1). The 5-year overall survival rate was 40% (95% CI: 31.2, 48.4) in the combination arm versus 33% (95% CI: 25.0, 41.0) in the dabrafenib monotherapy arm for patients who had a normal lactate dehydrogenase level at baseline, and 16% (95% CI: 8.4, 26.0) in the combination arm versus 14% (95% CI: 6.8, 23.1) in the dabrafenib monotherapy arm for patients with an elevated lactate dehydrogenase level at baseline.
Table 6. Overall Survival results for Study MEK115306 (COMBI-d):
| OS analysis (data cut-off: 12-Jan-2015) | 5-year OS analysis (data cut-off: 10-Dec-2018) | |||
|---|---|---|---|---|
| Dabrafenib + Trametinib (n=211) | Dabrafenib + Placebo (n=212) | Dabrafenib + Trametinib (n=211) | Dabrafenib + Placebo (n=212) | |
| Number of patients | ||||
| Died (event), n (%) | 99 (47) | 123 (58) | 135 (64) | 151 (71) |
| Estimates of OS (months) | ||||
| Median (95% CI) | 25.1 (19.2, NR) | 18.7 (15.2, 23.7) | 25.8 (19.2, 38.2) | 18.7 (15.2, 23.1) |
| Hazard ratio (95% CI) | 0.71 (0.55, 0.92) | 0.80 (0.63, 1.01) | ||
| p-value | 0.011 | NA | ||
| Overall survival estimate, % (95% CI) | Dabrafenib + Trametinib (n=211) | Dabrafenib + Placebo (n=212) | ||
| At 1 year | 74 (66.8, 79.0) | 68 (60.8, 73.5) | ||
| At 2 years | 52 (44.7, 58.6) | 42 (35.4, 48.9) | ||
| At 3 years | 43 (36.2, 50.1) | 31 (25.1, 37.9) | ||
| At 4 years | 35 (28.2, 41.8) | 29 (22.7, 35.2) | ||
| At 5 years | 32 (25.1, 38.3) | 27 (20.7, 33.0) | ||
NR = Not reached, NA = Not applicable
Figure 1. Kaplan-Meier overall survival curves for Study MEK115306 (ITT population):
Improvements for the primary endpoint of PFS were sustained over a 5 year timeframe in the combination arm compared to dabrafenib monotherapy. Improvements were also observed for overall response rate (ORR) and a longer duration of response (DoR) was observed in the combination arm compared to dabrafenib monotherapy (Table 7).
Table 7. Efficacy results for Study MEK115306 (COMBI-d):
| Primary analysis (data cut- off: 26-Aug-2013) | Updated analysis (data cut- off: 12-Jan-2015) | 5-year analysis (data cut- off: 10-Dec-2018) | ||||
|---|---|---|---|---|---|---|
| Endpoint | Dabrafenib + Trametinib (n=211) | Dabrafenib + Placebo (n=212) | Dabrafenib + Trametinib (n=211) | Dabrafenib + Placebo (n=212) | Dabrafenib + Trametinib (n=211) | Dabrafenib + Placebo (n=212) |
| PFSa | ||||||
| Progressive disease or death, n (%) | 102 (48) | 109 (51) | 139 (66) | 162 (76) | 160 (76) | 166 (78) |
| Median PFS (months) (95% CI) | 9.3 (7.7, 11.1) | 8.8 (5.9, 10.9) | 11.0 (8.0, 13.9) | 8.8 (5.9, 9.3) | 10.2 (8.1, 12.8) | 8.8 (5.9, 9.3) |
| Hazard Ratio (95% CI) | 0.75 (0.57, 0.99) | 0.67 (0.53, 0.84) | 0.73 (0.59, 0.91) | |||
| P value | 0.035 | <0.001f | NA | |||
| ORRb % (95% CI) | 67 (59.9, 73.0) | 51 (44.5, 58.4) | 69 (61.8, 74.8) | 53 (46.3, 60.2) | 69 (62.5, 75.4) | 54 (46.8, 60.6) |
| ORR difference (95% CI) | 15e (5.9, 24.5) | 15e (6.0, 24.5) | NA | |||
| P value | 0.0015 | 0.0014f | NA | |||
| DoRc (months) Median (95% CI) | 9.2d (7.4, NR) | 10.2d (7.5, NR) | 12.9 (9.4,19.5) | 10.6 (9.1, 13.8) | 12.9 (9.3, 18.4) | 10.2 (8.3, 13.8) |
a Progression-free survival (investigator assessed).
b Overall Response Rate = Complete Response + Partial Response
c Duration of response.
d At the time of the reporting the majority (≥59%) of investigator-assessed responses were still ongoing.
e ORR difference calculated based on the ORR result not rounded.
f Updated analysis was not pre-planned and the p-value was not adjusted for multiple testing.
NR = Not reached
NA = Not applicable
MEK116513 (COMBI-v):
Study MEK116513 was a 2-arm, randomised, open-label, Phase III study comparing dabrafenib and trametinib combination therapy with vemurafenib monotherapy in BRAF V600 mutation-positive unresectable or metastatic melanoma. The primary endpoint of the study was OS with a key secondary endpoint of PFS. Subjects were stratified by lactate dehydrogenase (LDH) level (> the upper limit of normal (ULN) versus ULN) and BRAF mutation (V600E versus V600K).
A total of 704 subjects were randomised 1:1 to either combination or vemurafenib. Most subjects were Caucasian (>96%) and male (55%), with a median age of 55 years (24% were ≥65 years). The majority of subjects had Stage IV M1c disease (61% overall). Most subjects had LDH ≤ULN (67%), ECOG performance status of 0 (70%), and visceral disease (78%) at baseline. Overall, 54% of subjects had <3 disease sites at baseline. The majority of subjects had BRAF V600E mutation-positive melanoma (89%). Subjects with brain metastases were not included in the trial.
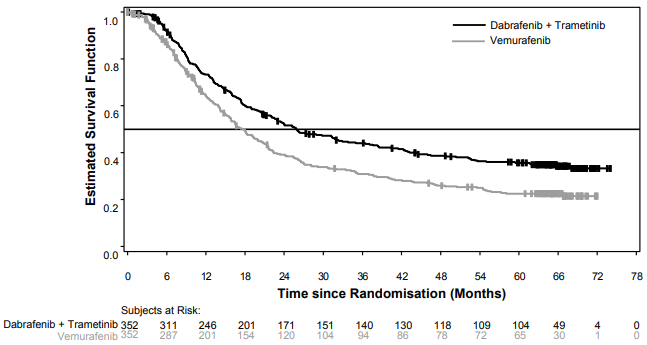
Median OS and estimated 1-year, 2-year, 3-year, 4-year and 5-year survival rates are presented in Table 8. From an OS analysis at 5 years, the median OS for the combination arm was approximately 8 months longer than the median OS for vemurafenib monotherapy (26.0 months versus 17.8 months) with 5-year survival rates of 36% for the combination versus 23% for vemurafenib monotherapy (Table 8, Figure 2). The Kaplan-Meier OS curve appears to stabilise from 3 to 5 years (see Figure 2).
The 5-year overall survival rate was 46% (95% CI: 38.8, 52.0) in the combination arm versus 28% (95% CI: 22.5, 34.6) in the vemurafenib monotherapy arm for patients who had a normal lactate dehydrogenase level at baseline, and 16% (95% CI: 9.3, 23.3) in the combination arm versus 10% (95% CI: 5.1, 17.4) in the vemurafenib monotherapy arm for patients with an elevated lactate dehydrogenase level at baseline.
Table 8. Overall Survival results for Study MEK116513 (COMBI-v):
| OS analysis data cut-off: 13-Mar-2015) | 5-year OS analysis (data cut-off: 08-Oct-2018) | |||
|---|---|---|---|---|
| Dabrafenib + Trametinib (n=352) | Vemurafenib (n=352) | Dabrafenib + Trametinib (n=352) | Vemurafenib (n=352) | |
| Number of patients | ||||
| Died (event), n (%) | 155 (44) | 194 (55) | 216 (61) | 246 (70) |
| Estimates of OS (months) | ||||
| Median (95% CI) | 25.6 (22.6, NR) | 18.0 (15.6, 20.7) | 26.0 (22.1, 33.8) | 17.8 (15.6, 20.7) |
| Adjusted hazard ratio (95% CI) | 0.66 (0.53, 0.81) | 0.70 (0.58, 0.84) | ||
| p-value | <0.001 | NA | ||
| Overall survival estimate, % (95% CI) | Dabrafenib + Trametinib (n=352) | Vemurafenib (n=352) | ||
| At 1 year | 72 (67, 77) | 65 (59, 70) | ||
| At 2 years | 53 (47.1, 57.8) | 39 (33.8, 44.5) | ||
| At 3 years | 44 (38.8, 49.4) | 31 (25.9, 36.2) | ||
| At 4 years | 39 (33.4, 44.0) | 26 (21.3, 31.0) | ||
| At 5 years | 36 (30.5, 40.9) | 23 (18.1, 27.4) | ||
NR = Not reached, NA = Not applicable
Figure 2. Kaplan-Meier curves Updated OS analysis for Study MEK116513:
Improvements for the secondary endpoint of PFS were sustained over a 5 year timeframe in the combination arm compared to vemurafenib monotherapy. Improvements were also observed for ORR and a longer DoR was observed in the combination arm compared to vemurafenib monotherapy (Table 9).
Table 9. Efficacy results for Study MEK116513 (COMBI-v):
| Primary analysis (Data cut-off: 17-Apr-2014) | 5-year analysis (Data cut-off: 08-Oct-2018) | |||
|---|---|---|---|---|
| Endpoint | Dabrafenib + Trametinib (n=352) | Vemurafenib (n=352) | Dabrafenib + Trametinib (n=352) | Vemurafenib (n=352) |
| PFSa | ||||
| Progressive disease or death, n (%) | 166 (47) | 217 (62) | 257 (73) | 259 (74) |
| Median PFS (months) (95% CI) | 11.4 (9.9, 14.9) | 7.3 (5.8, 7.8) | 12.1 (9.7, 14.7) | 7.3 (6.0, 8.1) |
| Hazard Ratio (95% CI) | 0.56 (0.46, 0.69) | 0.62 (0.52, 0.74) | ||
| P value | <0.001 | NA | ||
| ORRb % (95% CI) | 64 (59.1, 69.4) | 51 (46.1, 56.8) | 67 (62.2, 72.2) | 53 (47.2, 57.9) |
| ORR difference (95% CI) | 13 (5.7, 20.2) | NA | ||
| P value | 0.0005 | NA | ||
| DoRc (months) Median (95% CI) | 13.8d (11.0, NR) | 7.5d (7.3, 9.3) | 13.8 (11.3, 18.6) | 8.5 (7.4, 9.3) |
a Progression-free survival (investigator assessed)
b Overall Response Rate = Complete Response + Partial Response
c Duration of response
d At the time of the reporting the majority (59% of dabrafenib+trametinib and 42% of vemurafenib) of investigator-assessed responses were still ongoing
NR = Not reached
NA = Not applicable
Prior BRAF inhibitor therapy
There are limited data in patients taking the combination of trametinib with dabrafenib who have progressed on a prior BRAF inhibitor.
Part B of study BRF113220 included a cohort of 26 patients that had progressed on a BRAF inhibitor. The trametinib 2 mg once daily and dabrafenib 150 mg twice daily combination demonstrated limited clinical activity in patients who had progressed on a BRAF inhibitor (see section 4.4). The investigator-assessed confirmed response rate was 15% (95% CI: 4.4, 34.9) and the median PFS was 3.6 months (95% CI: 1.9, 5.2). Similar results were seen in the 45 patients who crossed over from dabrafenib monotherapy to the trametinib 2 mg once daily and dabrafenib 150 mg twice daily combination in Part C of this study. In these patients a 13% (95% CI: 5.0, 27.0) confirmed response rate was observed with a median PFS of 3.6 months (95% CI: 2, 4).
Patients with brain metastases
The efficacy and safety of trametinib in combination with dabrafenib in patients with BRAF mutant-positive melanoma that has metastasised to the brain was studied in a non-randomised, open-label, multicentre, Phase II study (COMBI-MB study). A total of 125 patients were enrolled into four cohorts:
- Cohort A: patients with BRAFV600E mutant melanoma with asymptomatic brain metastases without prior local brain-directed therapy and ECOG performance status of 0 or 1.
- Cohort B: patients with BRAFV600E mutant melanoma with asymptomatic brain metastases with prior local brain-directed therapy and ECOG performance status of 0 or1.
- Cohort C: patients with BRAFV600D/K/R mutant melanoma with asymptomatic brain metastases, with or without prior local brain-directed therapy and ECOG performance status of 0 or 1.
- Cohort D: patients with BRAFV600D/E/K/R mutant melanoma with symptomatic brain metastases, with or without prior local brain-directed therapy and ECOG performance status of 0 or 1 or 2.
The primary endpoint of the study was intracranial response in Cohort A, defined as the percentage of patients with a confirmed intracranial response assessed by the investigator using modified Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Intracranial response assessed by the investigator in Cohorts B, C and D were secondary endpoints of the study. Due to small sample size reflected by wide 95% CIs, the results in Cohorts B, C, and D should be interpreted with caution. Efficacy results are summarised in Table 10.
Table 10. Efficacy data by investigator assessment from COMBI-MB study:
| All treated patients population | ||||
|---|---|---|---|---|
| Endpoints/ assessment | Cohort A N=76 | Cohort B N=16 | Cohort C N=16 | Cohort D N=17 |
| Intracranial response rate, % (95 % CI) | ||||
| 59% (47.3, 70.4) | 56% (29.9, 80.2) | 44% (19.8, 70.1) | 59% (32.9, 81.6) | |
| Duration of intracranial response, median, months (95% CI) | ||||
| 6.5 (4.9, 8.6) | 7.3 (3.6, 12.6) | 8.3 (1.3, 15.0) | 4.5 (2.8, 5.9) | |
| Overall response rate, % (95% CI) | ||||
| 59% (47.3, 70.4) | 56% (29.9, 80.2) | 44% (19.8, 70.1) | 65% (38.3, 85.8) | |
| Progression-free survival, median, months (95% CI) | ||||
| 5.7 (5.3, 7.3) | 7.2 (4.7, 14.6) | 3.7 (1.7, 6.5) | 5.5 (3.7, 11.6) | |
| Overall survival, median, months (95% CI) | ||||
| 10.8 (8.7, 17.9) | 24.3 (7.9, NR) | 10.1 (4.6, 17.6) | 11.5 (6.8, 22.4) | |
CI = Confidence Interval
NR = Not reached
Trametinib monotherapy
Treatment naïve patients
The efficacy and safety of trametinib in patients with BRAF unresectable or metastatic mutant melanoma (V600E and V600K) were evaluated in a randomised open-label Phase III study (MEK114267 [METRIC]). Measurement of patients' BRAF V600 mutation status was required.
Patients (N=322) who were treatment naïve or may have received one prior chemotherapy treatment in the metastatic setting [Intent to Treat (ITT) population] were randomised 2:1 to receive trametinib 2 mg once daily or chemotherapy (dacarbazine 1000 mg/m² every 3 weeks or paclitaxel 175 mg/m² every 3 weeks). Treatment for all patients continued until disease progression, death or withdrawal.
The primary endpoint of the study was to evaluate the efficacy of trametinib compared to chemotherapy with respect to PFS in patients with advanced/metastatic BRAF V600E/K mutation-positive melanoma without a prior history of brain metastases (N=273) which is considered the primary efficacy population. The secondary endpoints were PFS in the ITT population and OS, ORR, and DoR in the primary efficacy population and ITT population. Patients in the chemotherapy arm were allowed to cross over to the trametinib arm after independent confirmation of progression. Of the patients with confirmed disease progression in the chemotherapy arm, a total of 51 (47%) crossed over to receive trametinib.
Baseline characteristics were balanced between treatment groups in the primary efficacy population and the ITT population. In the ITT population, 54% of patients were male and all were Caucasian. The median age was 54 years (22% were 65 years); all patients had an ECOG performance score of 0 or 1; and 3 % had history of brain metastases. Most patients (87%) in the ITT population had BRAF V600E mutation and 12% of patients had BRAF V600K. Most patients (66%) received no prior chemotherapy for advanced or metastatic disease.
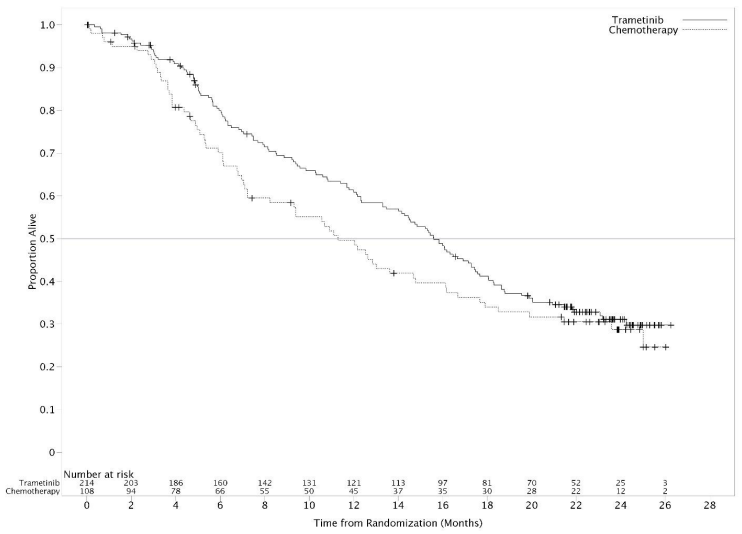
The efficacy results in the primary efficacy population were consistent with those in the ITT population; therefore, only the efficacy data for the ITT population are presented in Table 11. Kaplan-Meier curves of investigator-assessed OS (post-hoc analysis 20 May 2013) is presented in Figure 3.
Table 11. Investigator-assessed efficacy results (ITT population):
| Endpoint | Trametinib | Chemotherapya |
|---|---|---|
| Progression-Free Survival | (N=214) | (N=108) |
| Median PFS (months) (95% CI) | 4.8 (4.3, 4.9) | 1.5 (1.4, 2.7) |
| Hazard Ratio (95% CI) P value | 0.45 (0.33, 0.63) <0.0001 | |
| Overall Response Rate (%) | 22 | 8 |
ITT = Intent to Treat; PFS = Progression-free survival; CI = confidence interval.
a Chemotherapy included patients on dacarbazine (DTIC) 1000 mg/m² every 3 weeks or paclitaxel 175 mg/m² every 3 weeks.
The PFS result was consistent in the subgroup of patients with V600K mutation positive melanoma (HR=0.50; [95% CI: 0.18, 1.35], p=0.0788). An additional OS analysis was undertaken based upon the 20 May 2013 data cut, see Table 12.
For October 2011, 47% of subjects had crossed over, while for May 2013, 65% of subjects had crossed over.
Table 12. Survival data from the primary and post-hoc analyses:
| Cut-off dates | Treatment | Number of deaths (%) | Median months OS (95% CI) | Hazard ratio (95% CI) | Percent survival at 12 months (95% CI) |
|---|---|---|---|---|---|
| October 26, 2011 | Chemotherapy (n=108) | 29 (27) | NR | 0.54 (0.32, 0.92) | NR |
| Trametinib (n=214) | 35 (16) | NR | NR | ||
| May 20, 2013 | Chemotherapy (n=108) | 67 (62) | 11.3 (7.2, 14.8) | 0.78 (0.57, 1.06) | 50 (39,59) |
| Trametinib (n=214) | 137 (64) | 15.6 (14.0, 17.4) | 61 (54, 67) |
NR = not reached
Figure 3. Kaplan-Meier curves of overall survival (OS -ad hoc analysis 20 May 2013):
Prior BRAF inhibitor therapy
In a single-arm Phase II study, designed to evaluate the objective response rate, safety, and pharmacokinetics following dosing of trametinib at 2 mg once daily in patients with BRAF V600E, V600K, or V600D mutation-positive metastatic melanoma (MEK113583), two separate cohorts were enrolled: Cohort A: patients with prior treatment with a BRAF inhibitor either with or without other prior therapy, Cohort B: patients with at least 1 prior chemotherapy or immunotherapy, without prior treatment with a BRAF inhibitor.
In Cohort A of this study, trametinib did not demonstrate clinical activity in patients who had progressed on a prior BRAF inhibitor therapy.
Adjuvant treatment of Stage III melanoma
BRF115532 (COMBI-AD):
The efficacy and safety of trametinib in combination with dabrafenib were studied in a Phase III, multicentre, randomised, double-blind, placebo-controlled study in patients with Stage III (Stage IIIA [lymph node metastasis >1 mm], IIIB, or IIIC) cutaneous melanoma with a BRAF V600 E/K mutation, following complete resection.
Patients were randomised 1:1 to receive either combination therapy (dabrafenib 150 mg twice daily and trametinib 2 mg once daily) or two placebos for a period of 12 months. Enrollment required complete resection of melanoma with complete lymphadenectomy within 12 weeks prior to randomisation. Any prior systemic anti-cancer treatment, including radiotherapy, was not allowed. Patients with a history of prior malignancy, if disease-free for at least 5 years, were eligible. Patients presenting with malignancies with confirmed activating RAS mutations were not eligible. Patients were stratified by BRAF mutation status (V600E versus V600K) and stage of disease prior to surgery using the American Joint Committee on Cancer (AJCC) 7th edition Melanoma Staging System (by Stage III sub-stage, indicating different levels of lymph node involvement and primary tumour size and ulceration). The primary endpoint was investigator-assessed relapse-free survival (RFS), defined as the time from randomisation to disease recurrence or death from any cause. Radiological tumour assessment was conducted every 3 months for the first two years and every 6 months thereafter, until first relapse was observed. Secondary endpoints include overall survival (OS; key secondary endpoint), freedom from relapse (FFR) and distant metastasis-free survival (DMFS).
A total of 870 patients were randomised to the combination therapy (n=438) and placebo (n=432) arms. Most patients were Caucasian (99%) and male (55%), with a median age of 51 years (18% were ≥65 years). The study included patients with all sub-stages of Stage III disease prior to resection; 18% of these patients had lymph node involvement only identifiable by microscope and no primary tumour ulceration. The majority of patients had a BRAF V600E mutation (91%). At the time of the primary analysis, the median duration of follow-up (time from randomisation to last contact or death) was 2.83 years in the dabrafenib and trametinib combination arm and 2.75 years in the placebo arm.
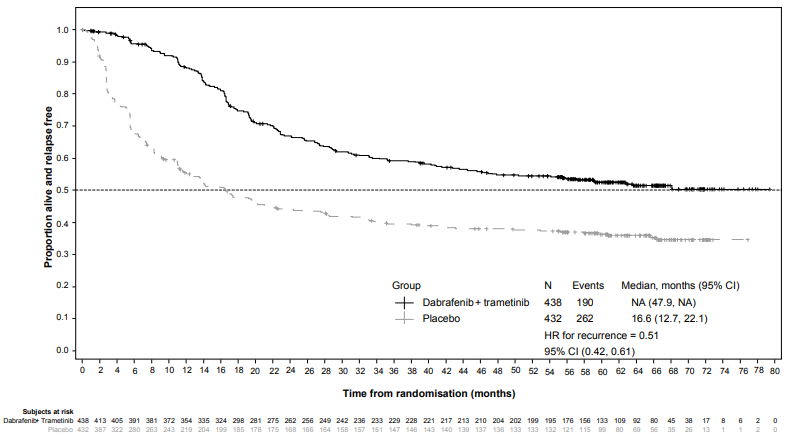
Results for the primary analysis of RFS are presented in Table 13. The study showed a statistically significant difference for the primary outcome of RFS between treatment arms, with a median RFS of 16.6 months for the placebo arm and not yet reached for the combination arm (HR: 0.47; 95% confidence interval: (0.39, 0.58); p=1.53×10-14). The observed RFS benefit was consistently demonstrated across subgroups of patients including age, sex and race. Results were also consistent across stratification factors for disease stage and BRAF V600 mutation type.
Table 13. Investigator-assessed RFS results for Study BRF115532 (COMBI-AD):
| RFS parameter | Dabrafenib + Trametinib N=438 | Placebo N=432 |
|---|---|---|
| Number of events, n (%) Recurrence Relapsed with distant metastasis Death | 166 (38%) 163 (37%) 103 (24%) 3 (<1%) | 248 (57%) 247 (57%) 133 (31%) 1 (<1%) |
| Median (months) (95% CI) | NE (44.5, NE) | 16.6 (12.7, 22.1) |
| Hazard ratio1 (95% CI) p-value2 | 0.47 (0.39, 0.58) 1.53×10-14 | |
| 1-year rate (95% CI) | 0.88 (0.85, 0.91) | 0.56 (0.51, 0.61) |
| 2-year rate (95% CI) | 0.67 (0.63, 0.72) | 0.44 (0.40, 0.49) |
| 3-year rate (95% CI) | 0.58 (0.54, 0.64) | 0.39 (0.35, 0.44) |
1 Hazard ratio is obtained from the stratified Pike model.
2 P-value is obtained from the two-sided stratified logrank test (stratification factors were disease stage – IIIA vs. IIIB vs. IIIC – and BRAF V600 mutation type – V600E vs. V600K)
NE = not estimable
Based on updated data with an additional 29 months of follow-up compared to the primary analysis (minimum follow-up of 59 months), the RFS benefit was maintained with an estimated HR of 0.51 (95% CI: (0.42, 0.61) (Figure 4). The 5-year RFS rate was 52% (95% CI: 48, 58) in the combination arm compared to 36% (95% CI: 32, 41) in the placebo arm.
Figure 4. Kaplan-Meier RFS curves for Study BRF115532 (ITT population, updated results):
Based on 153 events (60 [14%] in the combination arm and 93 [22%] in the placebo arm) corresponding to a 26% information fraction of the total target of 597 OS events, the estimated hazard ratio for OS was 0.57 (95% CI: 0.42, 0.79; p=0.0006). These results did not meet the pre-specified boundary to claim statistical significance at this first OS interim analysis (HR=0.50; p=0.000019). Survival estimates at 1 and 2 years from randomisation were 97% and 91% in the combination arm and 94% and 83% in the placebo arm, respectively.
Non-small cell lung cancer
Study BRF113928:
The efficacy and safety of trametinib in combination with dabrafenib was studied in a Phase II, three-cohort, multicentre, non-randomised and open-label study in which patients with Stage IV BRAF V600E mutant NSCLC were enrolled. The primary endpoint was ORR using the RECIST 1.1 assessed by the investigator. Secondary endpoints included DoR, PFS, OS, safety and population pharmacokinetics. ORR, DoR and PFS were also assessed by an Independent Review Committee (IRC) as a sensitivity analysis.
Cohorts were enrolled sequentially:
- Cohort A: Monotherapy (dabrafenib 150 mg twice daily), 84 patients enrolled. 78 patients had previous systemic treatment for their metastatic disease.
- Cohort B: Combination therapy (dabrafenib 150 mg twice daily and trametinib 2 mg once daily), 59 patients enrolled. 57 patients had 1-3 lines of previous systemic treatment for their metastatic disease. 2 patients had no previous systemic treatment and were included in the analysis for patients enrolled in Cohort C.
- Cohort C: Combination therapy (dabrafenib 150 mg twice daily and trametinib 2 mg once daily), 34 patients. All patients received study medicinal product as first-line treatment for metastatic disease.
Among the total of 93 patients who were enrolled in the combination therapy cohorts B and C, most patients were Caucasian (>90%), and similar female versus male (54% versus 46%), with a median age of 64 years in second-line or higher patients and 68 years in the first-line patients. Most patients (94%) enrolled in the combination-therapy-treated cohorts had an ECOG performance status of 0 or 1. 26 (28%) had never smoked. The majority of patients had a non-squamous histology. In the previously-treated population, 38 patients (67%) had one line of systemic anti-cancer therapy for metastatic disease.
At the time of the primary analysis, the primary endpoint of investigator-assessed ORR in the first-line population was 61.1% (95% CI, 43.5%, 76.9%), and in the previously-treated population was 66.7% (95% CI, 52.9%, 78.6%). These met the statistical significance to reject the null hypothesis that the ORR of dabrafenib in combination with trametinib for this NSCLC population was less than or equal to 30%. The ORR results assessed by IRC were consistent with the investigator assessment. The final analysis of efficacy performed 5 years after last subject first dose is presented in Table 14.
Table 14. Summary of efficacy in the combination treatment cohorts based on investigator and independent radiology review:
| Endpoint | Analysis | Combination 1st Line N=361 | Combination 2nd Line Plus N=571 |
|---|---|---|---|
| Overall confirmed response n (%) (95% CI) | By Investigator By IRC | 23 (63.9%) (46.2, 79.2) 23 (63.9%) (46.2, 79.2) | 39 (68.4%) (54.8, 80.1) 36 (63.2%) (49.3, 75.6) |
| Median DoR Months (95% CI) | By Investigator By IRC | 10.2 (8.3, 15.2) 15.2 (7.8, 23.5) | 9.8 (6.9, 18.3) 12.6 (5.8, 26.2) |
| Median PFS Months (95% CI) | By Investigator By IRC | 10.8 (7.0, 14.5) 14.6 (7.0, 22.1) | 10.2 (6.9, 16.7) 8.6 (5.2, 16.8) |
| Median OS Months (95% CI) | - | 17.3 (12.3, 40.2) | 18.2 (14.3, 28.6) |
1 Data cut-off: 7 January 2021
Other studies – pyrexia management analysis
Study CPDR001F2301 (COMBI-i) and Study CDRB436F2410 (COMBI-Aplus):
Pyrexia is observed in patients treated with dabrafenib and trametinib combination therapy. The initial registration studies for the combination therapy in the unresectable or metastatic melanoma setting (COMBI-d and COMBI-v; total N=559) and in the adjuvant melanoma setting (COMBI-AD, N=435) recommended to interrupt only dabrafenib in case of pyrexia (fever ≥38.5°C). In two subsequent studies in unresectable or metastatic melanoma (COMBI-i control arm, N=264) and in the adjuvant melanoma setting (COMBI-Aplus, N=552), interruption of both medicinal products when patient’s temperature is ≥38°C (COMBI-Aplus), or at the first symptom of pyrexia (COMBI-i; COMBI-Aplus for recurrent pyrexia) was advised. In COMBI-i and COMBI-Aplus there was a lower incidence of grade ¾ pyrexia, complicated pyrexia, hospitalisation due to serious pyrexia adverse events of special interest (AESIs), the time spent in pyrexia AESIs, and permanent discontinuations from both medicinal products due to pyrexia AESIs (the latter in the adjuvant setting only) compared to COMBI-d, COMBI-v and COMBI-AD. The COMBI-Aplus study met its primary endpoint with a composite rate of 8.0% (95% CI: 5.9, 10.6) for grade ¾ pyrexia, hospitalisation due to pyrexia, or permanent treatment discontinuation due to pyrexia compared to 20.0% (95% CI: 16.3, 24.1) for the historical control (COMBI-AD).
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with trametinib in all subsets of the paediatric population in melanoma and malignant neoplasms (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
Trametinib is absorbed orally with median time to achieve peak concentrations of 1.5 hours post-dose. The mean absolute bioavailability of a single 2 mg tablet dose is 72% relative to an intravenous (IV) microdose. The increase in exposure (Cmax and AUC) was dose-proportional following repeat dosing. Following administration of 2 mg once daily, steady-state geometric mean Cmax, AUC(0-τ) and predose concentration were 22.2 ng/ml, 370 ng*hr/ml and 12.1 ng/ml, respectively with a low peak:trough ratio (1.8). Inter-subject variability at steady state was low (<28%).
Trametinib accumulates with repeat daily dosing with a mean accumulation ratio of 6.0 at 2 mg once daily dose. Steady state was achieved by Day 15.
Administration of a single dose of trametinib with a high-fat, high-calorie meal resulted in a 70% and 10% decrease in Cmax and AUC, respectively compared to fasted conditions (see sections 4.2 and 4.5).
Distribution
Binding of trametinib to human plasma proteins is 97.4%. Trametinib has a volume of distribution of approximately 1200 L determined following administration of a 5 μg intravenous microdose.
Biotransformation
In vitro and in vivo studies demonstrated that trametinib is metabolised predominantly via deacetylation alone or in combination with mono-oxygenation. The deacetylated metabolite was further metabolised by glucuronidation. CYP3A4 oxidation is considered a minor pathway of metabolism. The deacetylation is mediated by the carboxyl-esterases 1b, 1c and 2, with possible contributions by other hydrolytic enzymes.
Following single and repeated doses of trametinib, trametinib as parent is the main circulating component in plasma.
Elimination
Mean terminal half-life is 127 hours (5.3 days) after single dose administration. Trametinib plasma IV clearance is 3.21 L/hr.
Total dose recovery was low after a 10-day collection period (<50%) following administration of a single oral dose of radiolabelled trametinib as a solution, due to the long elimination half-life. Drug-related material was excreted predominantly in the faeces (>80% of recovered radioactivity) and to a minor extent in urine (≤19%). Less than 0.1% of the excreted dose was recovered as parent in urine.
Special patient populations
Hepatic impairment
Population pharmacokinetic analyses and data from a clinical pharmacology study in patients with normal hepatic function or with mild, moderate or severe bilirubin and/or AST elevations (based on National Cancer Institute [NCI] classification) indicate that hepatic function does not significantly affect trametinib oral clearance.
Renal impairment
Renal impairment is unlikely to have a clinically relevant effect on trametinib pharmacokinetics given the low renal excretion of trametinib. The pharmacokinetics of trametinib were characterised in 223 patients enrolled in clinical trials with trametinib who had mild renal impairment and 35 patients with moderate renal impairment using a population pharmacokinetic analysis. Mild and moderate renal impairment had no effect on trametinib exposure (<6% for either group). No data are available in patients with severe renal impairment (see section 4.2).
Elderly
Based on the population pharmacokinetic analysis (range 19 to 92 years), age had no relevant clinical effect on trametinib pharmacokinetics. Safety data in patients ≥75 years is limited (see section 4.8).
Race
There are insufficient data to evaluate the potential effect of race on trametinib pharmacokinetics as clinical experience is limited to Caucasians.
Paediatric population
No studies have been conducted to investigate the pharmacokinetics of trametinib in paediatric patients.
Body weight and gender
Based on a population pharmacokinetic analysis, gender and body weight were found to influence trametinib oral clearance. Although smaller female subjects are predicted to have higher exposure than heavier male subjects, these differences are unlikely to be clinically relevant and no dosage adjustment is warranted.
Medicinal product interactions
Effects of trametinib on drug-metabolising enzymes and transporters: In vitro and in vivo data suggest that trametinib is unlikely to affect the pharmacokinetics of other medicinal products. Based on in vitro studies, trametinib is not an inhibitor of CYP1A2, CYP2A6, CYP2B6, CYP2D6 and CYP3A4. Trametinib was found to be an in vitro inhibitor of CYP2C8, CYP2C9 and CYP2C19, an inducer of CYP3A4 and an inhibitor of the transporters OAT1, OAT3, OCT2, MATE1, OATP1B1, OATP1B3, P-gp and BCRP. However, based on the low dose and low clinical systemic exposure relative to the in vitro potency of inhibition or induction values, trametinib is not considered to be an in vivo inhibitor or inducer of these enzymes or transporters, although transient inhibition of BCRP substrates in the gut may occur (see section 4.5).
Effects of other drugs on trametinib: In vivo and in vitro data suggest that the pharmacokinetics of trametinib are unlikely to be affected by other medicinal products. Trametinib is not a substrate of CYP enzymes or of the transporters BCRP, OATP1B1, OATP1B3, OATP2B1, OCT1, MRP2, and MATE1. Trametinib is an in vitro substrate of BSEP and the efflux transporter P-gp. Although trametinib exposure is unlikely to be affected by inhibition of BSEP, increased levels of trametinib upon strong inhibition of hepatic P-gp cannot be excluded (see section 4.5).
Effects of trametinib on other medicinal products: the effect of repeat-dose trametinib on the steady state pharmacokinetics of combination oral contraceptives, norethindrone and ethinyl estradiol, was assessed in a clinical study that consisted of 19 female patients with solid tumours. Norethindrone exposure increased by 20% and ethinyl estradiol exposure was similar when co-administered with trametinib. Based on these results, no loss of efficacy of hormonal contraceptives is expected when co-administered with trametinib monotherapy.
Preclinical safety data
Carcinogenicity studies with trametinib have not been conducted. Trametinib was not genotoxic in studies evaluating reverse mutations in bacteria, chromosomal aberrations in mammalian cells and micronuclei in the bone marrow of rats.
Trametinib may impair female fertility in humans, as in repeat-dose studies, increases in cystic follicles and decreases in corpora lutea were observed in female rats at exposures below the human clinical exposure based on AUC.
Additionally, in juvenile rats given trametinib, decreased ovarian weights, slight delays in hallmarks of female sexual maturation (vaginal opening and increased incidence of prominent terminal end buds within the mammary gland) and slight hypertrophy of the surface epithelium of the uterus were observed. All of these effects were reversible following an off-treatment period and attributable to pharmacology. However, in rat and dog toxicity studies up to 13 weeks in duration, there were no treatment effects observed in male reproductive tissues.
In embryo-foetal developmental toxicity studies in rats and rabbits, trametinib induced maternal and developmental toxicity. In rats decreased foetal weights and increased post-implantation loss were seen at exposures below or slightly above the clinical exposures based on AUC. In an embryo-foetal developmental toxicity study with rabbits, decreased foetal body weight, increased abortions, increased incidence of incomplete ossification and skeletal malformations were seen at sub-clinical exposures based on AUC).
In repeat-dose studies the effects seen after trametinib exposure are found mainly in the skin, gastrointestinal tract, haematological system, bone and liver. Most of the findings are reversible after drug-free recovery. In rats, hepatocellular necrosis and transaminase elevations were seen after 8 weeks at ≥0.062 mg/kg/day (approximately 0.8 times human clinical exposure based on AUC).
In mice, lower heart rate, heart weight and left ventricular function were observed without cardiac histopathology after 3 weeks at ≥0.25 mg/kg/day trametinib (approximately 3 times human clinical exposure based on AUC) for up to 3 weeks. In adult rats, mineralisation of multiple organs was associated with increased serum phosphorus and was closely associated with necrosis in heart, liver and kidney and haemorrhage in the lung at exposures comparable to the human clinical exposure. In rats, hypertrophy of the physis and increased bone turnover were observed, but the physeal hypertrophy is not expected to be clinically relevant for adult humans. In rats and dogs given trametinib at or below clinical exposures, bone marrow necrosis, lymphoid atrophy in thymus and GALT and lymphoid necrosis in lymph nodes, spleen and thymus were observed, which have the potential to impair immune function. In juvenile rats, increased heart weight with no histopathology was observed at 0.35 mg/kg/day (approximately twice the adult human clinical exposure based on AUC).
Trametinib was phototoxic in an in vitro mouse fibroblast 3T3 Neutral Red Uptake (NRU) assay at significantly higher concentrations than clinical exposures (IC50 at 2.92 µg/ml, ≥130 times the clinical exposure based on Cmax), indicating that there is low risk for phototoxicity to patients taking trametinib.
Combination with dabrafenib
In a study in dogs in which trametinib and dabrafenib were given in combination for 4 weeks, signs of gastro-intestinal toxicity and decreased lymphoid cellularity of the thymus were observed at lower exposures than in dogs given trametinib alone. Otherwise, similar toxicities were observed as in comparable monotherapy studies.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.