MEPACT Powder for concentrate for dispersion for infusion Ref.[9701] Active ingredients: Mifamurtide
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Takeda France SAS, 112 avenue Kléber, 75116, Paris, France
Therapeutic indications
MEPACT is indicated in children, adolescents and young adults for the treatment of high-grade resectable non-metastatic osteosarcoma after macroscopically complete surgical resection. It is used in combination with post-operative multi-agent chemotherapy. Safety and efficacy have been assessed in studies of patients 2 to 30 years of age at initial diagnosis (see section 5.1).
Posology and method of administration
Mifamurtide treatment should be initiated and supervised by specialist physicians experienced in the diagnosis and treatment of osteosarcoma.
Posology
The recommended dose of mifamurtide for all patients is 2 mg/m² body surface area. It should be administered as adjuvant therapy following resection: twice weekly at least 3 days apart for 12 weeks, followed by once-weekly treatments for an additional 24 weeks for a total of 48 infusions in 36 weeks.
Special populations
Adults >30 years
None of the patients treated in the osteosarcoma studies were 65 years or older and in the phase III randomised study, only patients up to the age of 30 years were included. Therefore, there are not sufficient data to recommend the use of MEPACT in patients >30 years of age.
Renal or hepatic impairment
There are no clinically meaningful effects of mild to moderate renal (creatinine clearance (CrCL) ≥30 mL/min) or hepatic impairment (Child-Pugh class A or B) on the pharmacokinetics of mifamurtide; therefore, dose adjustments are not necessary for these patients. However, as the variability in pharmacokinetics of mifamurtide is greater in subjects with moderate hepatic impairment (see section 5.2), and safety data in patients with moderate hepatic impairment is limited, caution when administering mifamurtide to patients with moderate hepatic impairment is recommended.
As no pharmacokinetic data of mifamurtide is available in patients with severe renal or hepatic impairment, caution when administering mifamurtide to these patients is recommended. Continued monitoring of the kidney and liver function is recommended if mifamurtide is used beyond completion of chemotherapy until all therapy is completed.
Paediatric population <2 years
The safety and efficacy of mifamurtide in children aged 0 to 2 years have not been established. No data are available.
Method of administration
MEPACT is administered by intravenous infusion over a period of 1 hour.
MEPACT must not be administered as a bolus injection.
For further instructions on reconstitution, filtering using the filter provided and dilution of the medicinal product before administration, see section 6.6.
Overdose
The maximum tolerated dose in phase I studies was 4-6 mg/m² with a high variability of adverse reactions. Signs and symptoms that were associated with higher doses and/or were dose limiting were not life-threatening, and included fever, chills, fatigue, nausea, vomiting, headache and hypoor hypertension.
A healthy adult volunteer accidentally received a single dose of 6.96 mg mifamurtide and experienced a reversible treatment-related event of orthostatic hypotension.
In the event of an overdose, it is recommended that appropriate supportive treatment be initiated. Supportive measures should be based on institutional guidelines and the clinical symptoms observed. Examples include paracetamol for fever, chills and headache and anti-emetics (other than steroids) for nausea and vomiting.
Shelf life
Shelf life
Unopened vial of powder: 30 months.
Reconstituted suspension: Chemical and physical stability has been demonstrated for 6 hours up to 25ºC.
From a microbiological point of view, immediate use is recommended. If not used immediately, the reconstituted, filtered and diluted solution in-use storage times and conditions prior to use of the reconstituted product must not be longer than 6 hours at 25ºC.
Do not refrigerate or freeze the solution.
Special precautions for storage
Store in a refrigerator (2°C-8°C). Do not freeze.
Keep the vial in the outer carton in order to protect from light.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
Nature and contents of container
50 mL type I glass vial with a grey butyl rubber stopper, aluminium seal and plastic flip-off cap, containing 4 mg of mifamurtide.
Each carton contains 1 vial and 1 single-use, non-pyrogenic, sterile filter for MEPACT supplied in a PVC-grade blister.
Special precautions for disposal and other handling
MEPACT must be reconstituted, filtered using the filter provided and further diluted using aseptic technique, prior to administration.
Each vial should be reconstituted with 50 mL of sodium chloride 9 mg/mL (0.9%) solution for injection. After reconstitution, each mL suspension in the vial contains 0.08 mg mifamurtide. The volume of reconstituted suspension corresponding to the calculated dose is extracted through the filter provided and further diluted with additional 50 mL sodium chloride 9 mg/mL (0.9%) solution for injection according to the detailed instructions shown below.
The reconstituted, filtered and diluted suspension for infusion is a homogenous, white to off-white, opaque liposomal suspension, free of visible particles and free of foam and lipid lumps.
Instructions for preparation of MEPACT for intravenous infusion
Materials provided in each package:
- MEPACT powder for concentrate for dispersion for infusion (vial)
- Filter for MEPACT
Materials required but not provided:
- Sodium chloride 9 mg/mL (0.9%) solution for injection, 100 mL bag
- 1 single use 60 or 100 mL sterile syringe with luer lock
- 2 medium (18) gauge sterile injection needles
It is recommended that the reconstitution of the liposomal suspension should be performed in a laminar flow cabinet utilising sterile gloves using aseptic technique.
The lyophilised powder should be allowed to reach a temperature between approximately 20°C-25°C prior to reconstitution, filtering using the filter provided and dilution. This should take approximately 30 minutes.
1. The cap of the vial should be removed and the stopper cleaned using an alcohol pad.
2. The filter should be removed from the blister pack, and the cap removed from the filter spike. The spike should then be inserted into the vial septum firmly until seated. The filter luer connector cap should not be removed at this time.
3. The 100 mL sodium chloride 9 mg/mL (0.9%) solution for injection bag, needle and syringe should be unpacked (not provided in the pack).
4. The site of the sodium chloride 9 mg/mL (0.9%) solution for injection bag where the needle is going to be inserted should be swabbed with an alcohol pad.
5. Using the needle and syringe, 50 mL of sodium chloride 9 mg/mL (0.9%) solution for injection should be withdrawn from the bag.
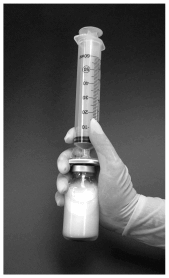
6. After removing the needle from the syringe, the syringe should be attached to the filter by opening the filter luer connector cap (figure 1).
Figure 1:
7. The sodium chloride 9 mg/mL (0.9%) solution for injection is added to the vial by slow, firm depression of the syringe plunger. The filter and syringe must not be removed from the vial.
8. The vial should be allowed to stand undisturbed for 1 minute to ensure thorough hydration of the dry substance.
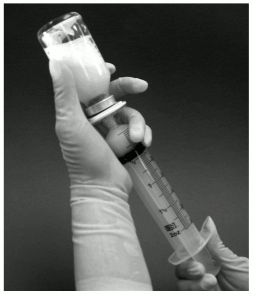
9. The vial should then be shaken vigorously for 1 minute while keeping the filter and syringe attached. During this time the liposomes are formed spontaneously (figure 2).
Figure 2:
10. The desired dose may be withdrawn from the vial by inverting the vial and slowly pulling back on the syringe plunger (figure 3). Each mL reconstituted suspension contains 0.08 mg mifamurtide. The volume of suspension to be withdrawn for dose quantities is calculated as follows:
Volume to withdraw = [12.5 x calculated dose (mg)] mL
For convenience, the following table of concordance is provided:
| Dose | Volume |
|---|---|
| 1.0 mg | 12.5 ml |
| 2.0 mg | 25 ml |
| 3.0 mg | 37.5 ml |
| 4.0 mg | 50 ml |
Figure 3:
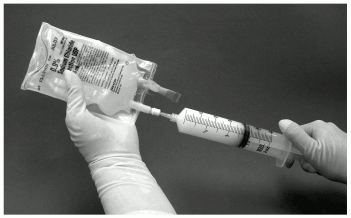
11. The syringe should then be removed from the filter and a new needle placed on the suspension-filled syringe. The bag injection site should be wiped with an alcohol pad and the suspension in the syringe should be injected into the original bag containing the remaining 50 mL of sodium chloride 9 mg/mL (0.9%) solution for injection (figure 4).
Figure 4:
12. The bag should be gently swirled to mix the solution.
13. Patient identification, time and date should be added to the label on the bag containing the reconstituted, filtered and diluted liposomal suspension.
14. Chemical and physical in-use stability has been demonstrated for 6 hours at room temperature (between approximately 20°C-25°C).
15. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 6 hours at room temperature.
16. Based on the liposomal nature of the product, use of an infusion set with an in-line filter during administration is not recommended.
17. The liposomal suspension is infused intravenously over about 1 hour.
No special requirements for disposal.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.