MIGRANAL Nasal spray Ref.[10876] Active ingredients: Dihydroergotamine
Source: FDA, National Drug Code (US) Revision Year: 2019
2. Clinical Pharmacology
Mechanism of Action
Dihydroergotamine binds with high affinity to 5-HT1Dα and 5-HT1Dβ receptors. It also binds with high affinity to serotonin 5-HT1A, 5-HT2A, and 5-HT2C receptors, noradrenaline α2A, α2B and α1 receptors, and dopamine D2L and D3 receptors.
The therapeutic activity of dihydroergotamine in migraine is generally attributed to the agonist effect at 5-HT1D receptors. Two current theories have been proposed to explain the efficacy of 5-HT1D receptor agonists in migraine. One theory suggests that activation of 5-HT1D receptors located on intracranial blood vessels, including those on arterio-venous anastomoses, leads to vasoconstriction, which correlates with the relief of migraine headache. The alternative hypothesis suggests that activation of 5-HT1D receptors on sensory nerve endings of the trigeminal system results in the inhibition of pro-inflammatory neuropeptide release. In addition, dihydroergotamine possesses oxytocic properties (See CONTRAINDICATIONS).
Pharmacokinetics
Absorption
Dihydroergotamine mesylate is poorly bioavailable following oral administration. Following intranasal administration, however, the mean bioavailability of dihydroergotamine mesylate is 32% relative to the injectable administration. Absorption is variable, probably reflecting both intersubject differences of absorption and the technique used for self-administration.
Distribution
Dihydroergotamine mesylate is 93% plasma protein bound. The apparent steady-state volume of distribution is approximately 800 liters.
Metabolism
Four dihydroergotamine mesylate metabolites have been identified in human plasma following oral administration. The major metabolite, 8'-β-hydroxydihydroergotamine, exhibits affinity equivalent to its parent for adrenergic and 5-HT receptors and demonstrates equivalent potency in several venoconstrictor activity models, in vivo and in vitro. The other metabolites, i.e., dihydrolysergic acid, dihydrolysergic amide and a metabolite formed by oxidative opening of the proline ring are of minor importance. Following nasal administration, total metabolites represent only 20%-30% of plasma AUC. The systemic clearance of dihydroergotamine mesylate following I.V. and I.M. administration is 1.5 L/min. Quantitative pharmacokinetic characterization of the four metabolites has not been performed.
Excretion
The major excretory route of dihydroergotamine is via the bile in the feces. After intranasal administration the urinary recovery of parent drug amounts to about 2% of the administered dose compared to 6% after I.M. administration. The total body clearance is 1.5 L/min which reflects mainly hepatic clearance. The renal clearance (0.1 L/min) is unaffected by the route of dihydroergotamine administration. The decline of plasma dihydroergotamine is biphasic with a terminal half-life of about 10 hours.
Subpopulations
No studies have been conducted on the effect of renal or hepatic impairment, gender, race, or ethnicity on dihydroergotamine pharmacokinetics. MIGRANAL (dihydroergotamine mesylate) Nasal Spray is contraindicated in patients with severely impaired hepatic or renal function (See CONTRAINDICATIONS).
Interactions
The pharmacokinetics of dihydroergotamine did not appear to be significantly affected by the concomitant use of a local vasoconstrictor (e.g., fenoxazoline).
Multiple oral doses of the β-adrenoceptor antagonist propranolol, used for migraine prophylaxis, had no significant influence on the Cmax, Tmax or AUC of dihydroergotamine doses up to 4 mg. Pharmacokinetic interactions have been reported in patients treated orally with other ergot alkaloids (e.g., increased levels of ergotamine) and macrolide antibiotics, principally troleandomycin, presumably due to inhibition of cytochrome P450 3A metabolism of the alkaloids by troleandomycin. Dihydroergotamine has also been shown to be an inhibitor of cytochrome P450 3A catalyzed reactions and rare reports of ergotism have been obtained from patients treated with dihydroergotamine and macrolide antibiotics (e.g., troleandomycin, clarithromycin, erythromycin), and in patients treated with dihydroergotamine and protease inhibitors (e.g. ritonavir), presumably due to inhibition of cytochrome P450 3A metabolism of ergotamine (See CONTRAINDICATIONS). No pharmacokinetic interactions involving other cytochrome P450 isoenzymes are known.
Clinical Trials
The efficacy of MIGRANAL (dihydroergotamine mesylate) Nasal Spray for the acute treatment of migraine headaches was evaluated in four randomized, double blind, placebo controlled studies in the U.S. The patient population for the trials was predominantly female (87%) and Caucasian (95%) with a mean age of 39 years (range 18 to 65 years). Patients treated a single moderate to severe migraine headache with a single dose of study medication and assessed pain severity over the 24 hours following treatment. Headache response was determined 0.5, 1, 2, 3 and 4 hours after dosing and was defined as a reduction in headache severity to mild or no pain. In studies 1 and 2, a four-point pain intensity scale was utilized; in studies 3 and 4, a five-point scale was used that included both pain response and restoration of function for "severe" or "incapacitating" pain, a less clear endpoint. Although rescue medication was allowed in all four studies, patients were instructed not to use them during the four hour observation period. In studies 3 and 4, a total dose of 2 mg was compared to placebo. In studies 1 and 2, doses of 2 and 3 mg were evaluated, and showed no advantage of the higher dose for a single treatment. In all studies, patients received a regimen consisting of 0.5 mg in each nostril, repeated in 15 minutes (and again in another 15 minutes for the 3 mg dose in studies 1 and 2).
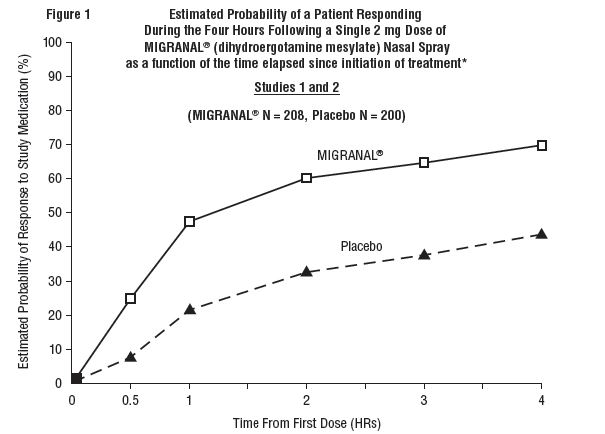
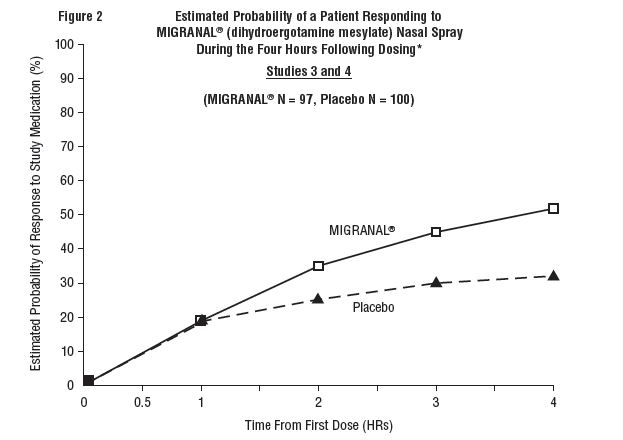
The percentage of patients achieving headache response 4 hours after treatment was significantly greater in patients receiving 2 mg doses of MIGRANAL (dihydroergotamine mesylate) Nasal Spray compared to those receiving placebo in 3 of the 4 studies (see Tables 1 & 2 and Figures 1 & 2).
Table 1. Studies 1 and 2: Percentage of patients with headache response* 2 and 4 hours following a single treatment of study medication [MIGRANAL (dihydroergotamine mesylate) Nasal Spray or Placebo]:
| N | 2 hours | 4 hours | ||
|---|---|---|---|---|
| Study 1 | MIGRANAL | 105 | 61%† | 70%† |
| Placebo | 98 | 23% | 28% | |
| Study 2 | MIGRANAL | 103 | 47% | 56%‡ |
| Placebo | 102 | 33% | 35% |
* Headache response was defined as a reduction in headache severity to mild or no pain. Headache response was based on pain intensity as interpreted by the patient using a fourpoint pain intensity scale.
† p value < 0.001
‡ p value < 0.01
Table 2. Studies 3 and 4: Percentage of patients with headache response* 2 and 4 hours following a single treatment of study medication [MIGRANAL (dihydroergotamine mesylate) Nasal Spray or Placebo]:
| N | 2 hours | 4 hours | ||
|---|---|---|---|---|
| Study 3 | MIGRANAL | 50 | 32% | 48%† |
| Placebo | 50 | 20% | 22% | |
| Study 4 | MIGRANAL | 47 | 30% | 47% |
| Placebo | 50 | 20% | 30% |
* Headache response was defined as a reduction in headache severity to mild or no pain. Headache response was evaluated on a five-point scale that included both pain response and restoration of function for "severe" or "incapacitating" pain.
† p value < 0.01
Comparisons of drug performance based upon results obtained in different clinical trials are never reliable. Because studies are conducted at different times, with different samples of patients, by different investigators, employing different criteria and/or different interpretations of the same criteria, under different conditions (dose, dosing regimen, etc.), quantitative estimates of treatment response and the timing of response may be expected to vary considerably from study to study.
The Kaplan-Meier plots below (Figures 1 & 2) provides an estimate of the probability that a patient will have responded to a single 2 mg dose of MIGRANAL (dihydroergotamine mesylate) Nasal Spray as a function of the time elapsed since initiation of treatment.
* The figure shows the probability over time of obtaining a response following treatment with MIGRANAL (dihydroergotamine mesylate) Nasal Spray. Headache response was based on pain intensity as interpreted by the patient using a four-point pain intensity scale. Patients not achieving response within 4 hours were censored to 4 hours.
* The figure shows the probability over time of obtaining a response following treatment with MIGRANAL (dihydroergotamine mesylate) Nasal Spray. Headache response was evaluated on a five-point scale that confounded pain response and restoration of function for "severe" or "incapacitating" pain. Patients not achieving response within 4 hours were censored to 4 hours.
For patients with migraine-associated nausea, photophobia, and phonophobia at baseline, there was a lower incidence of these symptoms at 2 and 4 hours following administration of MIGRANAL (dihydroergotamine mesylate) Nasal Spray compared to placebo.
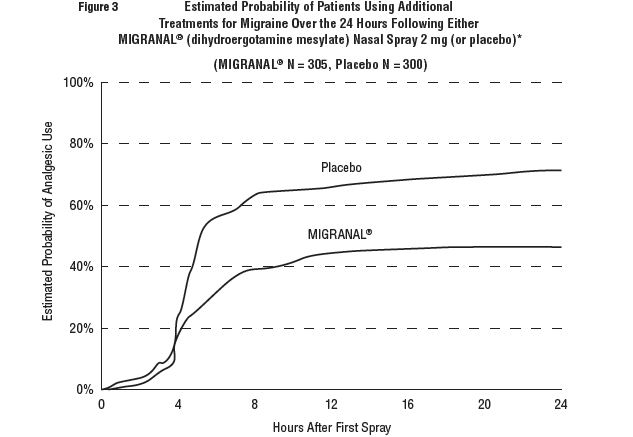
Patients were not allowed to use additional treatments for eight hours prior to study medication dosing and during the four hour observation period following study treatment. Following the 4 hour observation period, patients were allowed to use additional treatments. For all studies, the estimated probability of patients using additional treatments for their migraines over the 24 hours following the single 2 mg dose of study treatment is summarized in Figure 3 below.
* Kaplan-Meier plot based on data obtained from all studies with patients not using additional treatments censored to 24 hours. All patients received a single treatment of study medication for their migraine attack. The plot also includes patients who had no response to the initial dose.
Neither age nor sex appear to effect the patient's response to MIGRANAL (dihydroergotamine mesylate) Nasal Spray. While patients with menstrual migraine, migraine with aura, and migraine without aura by medical history were included in the clinical evaluation of MIGRANAL (dihydroergotamine mesylate) Nasal Spray, patients were not required to report the specific type of migraine treated with study medication. Thus, neither the effect of menses on migraine nor the presence or the absence of aura were assessed. The racial distribution of patients was insufficient to determine the effect of race on the efficacy of MIGRANAL (dihydroergotamine mesylate) Nasal Spray.
6.6. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Assessment of the carcinogenic potential of dihydroergotamine mesylate in mice and rats is ongoing.
Mutagenesis
Dihydroergotamine mesylate was clastogenic in two in vitro chromosomal aberration assays, the V79 Chinese hamster cell assay with metabolic activation and the cultured human peripheral blood lymphocyte assay. There was no evidence of mutagenic potential when dihydroergotamine mesylate was tested in the presence or absence of metabolic activation in two gene mutation assays (the Ames test and the in vitro mammalian Chinese hamster V79/HGPRT assay) and in an assay for DNA damage (the rat hepatocyte unscheduled DNA synthesis test). Dihydroergotamine was not clastogenic in the in vivo mouse and hamster micronucleus tests.
Impairment of Fertility
There was no evidence of impairment of fertility in rats given intranasal doses of MIGRANAL (dihydroergotamine mesylate) Nasal Spray up to 1.6 mg/day (associated with mean plasma dihydroergotamine mesylate exposures [AUC] approximately 9 to 11 times those in humans receiving the MRDD of 4 mg).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.