MOVANTIK Film-coated tablet Ref.[10882] Active ingredients: Naloxegol
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
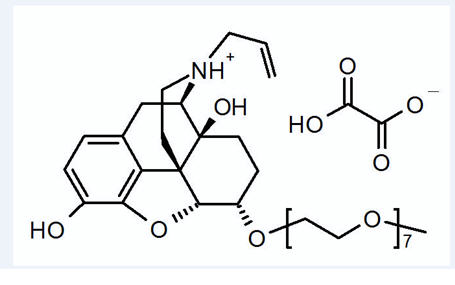
MOVANTIK (naloxegol), an opioid antagonist, contains naloxegol oxalate as the active ingredient. (Naloxegol is a PEGylated derivative of naloxone.)
The chemical name for naloxegol oxalate is: (5α,6α)-17-allyl-6-(2,5,8,11,14,17,20-heptaoxadocosan-22-yloxy)-4,5-epoxymorphinan-3,14-diol oxalate.
The structural formula is:
The empirical formula for naloxegol oxalate is C34H53NO11.C2H2O4 and the molecular weight is 742.
Naloxegol oxalate is a white to off-white powder, with high aqueous solubility across the physiologic pH range.
MOVANTIK (naloxegol) tablets for oral use contain 14.2 mg and 28.5 mg of naloxegol oxalate, respectively, equivalent to 12.5 mg and 25 mg of naloxegol.
Excipients in tablet core are: mannitol, cellulose microcrystalline, croscarmellose sodium, magnesium stearate, and propyl gallate.
Excipients in tablet coat are: hypromellose, titanium dioxide, polyethylene glycol, iron oxide red, and iron oxide black.
| Dosage Forms and Strengths |
|---|
|
MOVANTIK (naloxegol) is available in two strengths:
|
| How Supplied |
|---|
|
MOVANTIK (naloxegol) tablets are supplied as:
MOVANTIK is a registered trademark of the AstraZeneca Group of companies and used under license by RedHill Biopharma Inc. Distributed by: RedHill Biopharma Inc., Raleigh, NC 27617 |
Drugs
| Drug | Countries | |
|---|---|---|
| MOVANTIK | Canada, New Zealand, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.