MUCOSTA Tablet Ref.[50979] Active ingredients: Rebamipide
Source: Web Search Publisher: Manufactured By: Korea Otsuka Pharmaceutical Co., Ltd., Gyeonggi-do, Korea Under Authority of: Otsuka Pharmaceutical Co., Ltd., Japan Marketed By: Otsuka Pakistan Limited (A company of Otsuka Group Japan) ...
Product name and form
Mucosta.
| Pharmaceutical Form | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||
Qualitative and quantitative composition
MUCOSTA Tablets 100:
Each MUCOSTA Tablet contains 100 mg of rebamipide and the following inactive ingredients: Microcrystalline cellulose, Low Substituted Hydroxypropylcellulose, hydroxypropylcellulose, magnesium stearate, hypromellose, macrogol 6000, and titanium oxide.
| Active Ingredient |
|---|
|
Rebamipide, a gastroprotective drug, is a compound selected from over 500 amino acid analogs of 2(1H)-quinolinone. Rebamipide stimulates prostaglandin generation in gastric mucosa and improves not only the speed but also the quality of ulcer healing. Rebamipide works by enhancing mucosal defense, scavenging free radicals and temporarily activating genes encoding cyclooxygenase-2. |
| List of Excipients |
|---|
|
|
Pack sizes and marketing
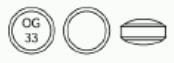
Mucosta tablets are round, white, film coated tablets, with a code OG33 and supplied as 100mg tablets in packs of 100 tablets (blister strips of 10 × 10).
Marketing authorization holder
Manufactured By: Korea Otsuka Pharmaceutical Co., Ltd., Gyeonggi-do, Korea
Under Authority of: Otsuka Pharmaceutical Co., Ltd., Japan
Marketed By: Otsuka Pakistan Limited (A company of Otsuka Group Japan)
Drugs
| Drug | Countries | |
|---|---|---|
| MUCOSTA | Japan |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.