MULTIBIC Solution for haemodialysis/haemofiltration Ref.[50679] Active ingredients: Calcium chloride Glucose Magnesium chloride Potassium chloride Sodium bicarbonate Sodium chloride
Source: Marketing Authorisation Holder Revision Year: 2017 Publisher: Fresenius Medical Care Deutschland GmbH, Else-Kröner-Straße 1, 61352 Bad Homburg v.d.H., Germany
4.1. Therapeutic indications
multiBic potassium-free/2/3/4 mmol/l potassium is indicated for intravenous use as substitution solution in haemofiltration and haemodiafiltration, and as dialysis solution in haemodialysis and haemodiafiltration.
For use in patients:
- with acute kidney injury requiring continuous renal replacement therapy: continuous haemodialysis, haemofiltration or haemodiafiltration treatments.
- with chronic kidney disease in whom a transient treatment is indicated, e.g. during the stay on an intensive care unit.
- when continuous renal replacement therapy is indicated as part of the treatment of an intoxication with water soluble, filterable/dialyzable toxins.
multiBic potassium-free/2/3/4 mmol/l potassium is indicated in adults.
4.2. Posology and method of administration
Continuous renal replacement therapy including the prescription of this medicinal product should be performed under the direction of a physician with experience in these treatments.
Posology
In acute kidney injury, a continuous treatment with a dose of 2000 ml/h multiBic potassium-free/2/3/4 mmol/l potassium is appropriate in adults with a body weight of 70 kg to remove metabolic waste products depending on the metabolic status of the patient. The dose should be adapted to the body size of the patient.
In patients with chronic kidney disease, unless clinically indicated otherwise, the dose of multiBic potassium-free/2/3/4 mmol/l potassium should be at least one third of the body weight per session with three sessions applied per week. Increasing the volume applied per week or distributing this weekly volume to more than 3 treatments per week can be required.
The dose and the duration of haemodialysis, haemofiltration or haemodiafiltration necessary in treatment of acute states of intoxication depends on the toxin and its concentration and the severity of clinical symptoms and has to be clinically decided on the individual patient’s condition.
A maximum dose of 75 litre per day is recommended.
Paediatric population
The safety and efficacy of multiBic potassium-free/2/3/4 mmol/l potassium in children have not yet been established (see sections 4.4 and 5.1).
Method of administration
For intravenous use and haemodialysis.
For instructions on use of the product, see section 6.6.
4.9. Overdose
After use of recommended doses no reports of emergency situations have arisen; moreover, the administration of this medicinal product can be discontinued at any time. If fluid balance is not accurately calculated and monitored, hyperhydration or dehydration may occur, with the resultant associated circulatory reactions. These may be manifest through changes in blood pressure, central venous pressure, heart rate, and pulmonary arterial pressure. In cases of hyperhydration congestive cardiac failure and/or pulmonary congestion may be induced.
In cases of hyperhydration, net fluid removal should be increased on the device used for continuous renal replacement therapy. In cases of marked dehydration, net fluid removal by the device used for continuous renal replacement therapy should be decreased or discontinued; alternatively, fluid resuscitation can be applied to restore the hydration status.
If too large volume is applied, this may result in disturbances of electrolyte concentrations and the acid-base-balance, e. g. an overdose of bicarbonate may occur if an inappropriate large volume of the solution for haemodialysis/haemofiltration is infused/administered. This could possibly lead to metabolic alkalosis, decrease of ionized calcium, or tetany.
6.3. Shelf life
2 years.
Storage conditions after mixing of the two compartments (ready-to-use solution):
Chemical and physical in-use stability of the ready-to-use solution has been demonstrated for 48 hours at 30°C. It is not recommended to store the ready-to-use solution longer than 48 h including duration of treatment or at a temperature higher than 30°C prior to the inlet of the pump unit.
From a microbiological point of view, once connected to the haemodialysis, haemofiltration or haemodiafiltration circuit, and as hydrogen carbonate is present, the product shall be used immediately.
6.4. Special precautions for storage
Do not store below +4°C.
6.5. Nature and contents of container
Double chamber bag with 4750 ml (alkaline hydrogen carbonate solution) + 250 ml (acidic electrolyte, glucose solution) = 5000 ml (ready-to-use solution).
The foil used for the bag is made of polyethylene-terephthalate, SiOx, polyamide, and polyolefine.
Each bag is equipped with a HF-connector, a Luer-lock-connector and an injection port, and is covered by a protective foil.
Pack size: 2 bags of 5000 ml.
6.6. Special precautions for disposal and other handling
Do not use unless the ready-to-use solution is clear and colourless and the bag and connectors are undamaged.
For single use only. Any unused solution must be discarded.
Must be used by means of metering pumps.
The solution for haemodialysis/haemofiltration should be administered in three steps:
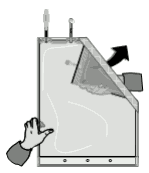
1. Removal of the protective foil and careful inspection of the bag
The protective foil should only be removed immediately before administration. Plastic containers may occasionally be damaged during transport from the manufacturer to the clinic or within the clinic itself. This can lead to contamination and microbiological or fungal growth in the solutions. Therefore, careful visual inspection of the bag and the solutions before mixing is necessary. Particular attention should be paid to even the slightest damage to the closure, the welded seam and the corners of the bag in view of a possible contamination.
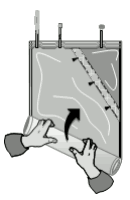
2. Mixing of the two compartments
The two-compartment-bag – the bicarbonate and the electrolytes including glucose compartments – are mixed immediately before use to obtain a ready-for-use solution.
A.
Unfold the small compartment.
B.
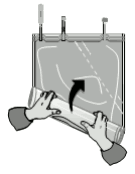
Roll up the solution bag starting from the corner opposite the small compartment...
C.
...until the peel seam between both compartments has opened along its entire length and the solutions from both compartments are mixed.
After mixing both compartments, it must be checked, that the peel seam is completely open, that the mixed solution is clear and colourless and that the bag is not leaking.
3. Application of the ready-to-use solution
The ready-to-use solution must be used immediately, but within a maximum of 48 hours after mixing.
Any admixture to the ready-to-use solution must only be done after the ready-to-use solution has been thoroughly mixed. After such an admixture, the ready-to-use solution should again be thoroughly mixed prior to use.
Admixtures of sodium chloride solution (concentration between 3% and 30% sodium chloride; up to 250 mmol sodium chloride per 5 litre multiBic solution) and water for injection (up to 1250 ml per 5 litre multiBic solution) are compatible with this medicinal product.
If not otherwise prescribed, the ready-to-use solution should be warmed immediately before use to 36.5°C – 38.0°C. The exact temperature must be selected depending on clinical requirements and the technical equipment used.
No special requirements for disposal.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.