NARCAN NALOXONE HCI Ref.[90494] Active ingredients: Naloxone
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
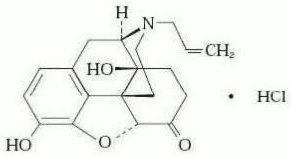
NARCAN (naloxone hydrochloride) Nasal Spray is a pre-filled, single dose intranasal spray. Chemically, naloxone hydrochloride is the hydrochloride salt of 17-Allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one hydrochloride with the following structure:
C19H21NO4•HCl
M.W. 363.84
Naloxone hydrochloride, an opioid antagonist, occurs as a white to slightly off-white powder, and is soluble in water, in dilute acids, and in strong alkali; slightly soluble in alcohol; practically insoluble in ether and in chloroform.
Each NARCAN Nasal Spray contains a 2 mg or 4 mg single dose of naloxone hydrochloride in a 0.1 mL (100 microliter) aqueous solution.
Inactive ingredients include benzalkonium chloride (preservative), disodium ethylenediaminetetraacetate (stabilizer), sodium chloride, hydrochloric acid to adjust pH, and purified water. The pH range is 3.5 to 5.5.
| Dosage Forms and Strengths |
|---|
|
NARCAN Nasal Spray is supplied as a single-dose intranasal spray containing 2 mg or 4 mg of naloxone hydrochloride in 0.1 mL. |
| How Supplied |
|---|
|
NARCAN Nasal Spray 2 mg is supplied as a carton containing four (4) blister packages (NDC 69547-212-04) each with a single spray device and as a carton containing twenty-four (24) blister packages (NDC 69547-212-24) each with a single spray device. NARCAN Nasal Spray 4 mg is supplied as carton containing two (2) blister packages (NDC 69547-353-02) each with a single spray device. NARCAN Nasal Spray is not made with natural rubber latex. Distributed by Adapt Pharma, Inc., Radnor, PA 19087 USA. |
Drugs
| Drug | Countries | |
|---|---|---|
| NARCAN | Australia, Brazil, Canada, France, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.