NERLYNX Film-coated tablet Ref.[10146] Active ingredients: Neratinib
Source: FDA, National Drug Code (US) Revision Year: 2024
Product description
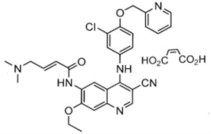
NERLYNX (neratinib) immediate release, film-coated tablets for oral administration contain 40 mg of neratinib, equivalent to 48.31 mg neratinib maleate. Neratinib is a member of the 4-anilino quinolidine class of protein kinase inhibitors. The molecular formula for neratinib maleate is C 30H 29ClN 6O 3•C 4H 4O 4 and the molecular weight is 673.11 Daltons.
The chemical name is (E)-N-{4-[3-chloro-4-(pyridin-2-ylmethoxy)anilino]-3-cyano-7-ethoxyquinolin-6-yl}-4-(dimethylamino)but-2-enamide maleate, and its structural formula is:
Neratinib maleate is an off-white to yellow powder with pKas of 7.65 and 4.66. The solubility of neratinib maleate increases dramatically as neratinib becomes protonated at acidic pH. Neratinib maleate is sparingly soluble at pH 1.2 (32.90 mg/mL) and insoluble at approximate pH 5.0 and above (0.08 mg/mL or less).
Inactive ingredients: Tablet Core: colloidal silicon dioxide, mannitol, microcrystalline cellulose, crospovidone, povidone, magnesium stearate, and purified water. Coating: red film coat: polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and iron oxide red.
| Dosage Forms and Strengths |
|---|
|
Tablets: 40 mg neratinib (equivalent to 48.31 mg of neratinib maleate). Film-coated, red, oval shaped and debossed with 'W104' on one side and plain on the other side. |
| How Supplied |
|---|
|
NERLYNX 40 mg film-coated tablets are red, oval shaped and debossed with 'W104' on one side and plain on the other side. NERLYNX is available in:
Manufactured for Puma Biotechnology, Inc., 10880 Wilshire Blvd., Suite 2150, Los Angeles, CA 90024-4106 |
Drugs
| Drug | Countries | |
|---|---|---|
| NERLYNX | Austria, Canada, Ecuador, Estonia, Finland, France, Hong Kong, Croatia, Ireland, Israel, Italy, Lithuania, Netherlands, New Zealand, Poland, Singapore, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.