NEXLETOL Film-coated tablet Ref.[9945] Active ingredients: Bempedoic acid
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Bempedoic acid is an adenosine triphosphate-citrate lyase (ACL) inhibitor that lowers low-density lipoprotein cholesterol (LDL-C) by inhibition of cholesterol synthesis in the liver. ACL is an enzyme upstream of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase in the cholesterol biosynthesis pathway. Bempedoic acid and its active metabolite, ESP15228, require coenzyme A (CoA) activation by very long-chain acyl-CoA synthetase 1 (ACSVL1) to ETC-1002-CoA and ESP15228-CoA, respectively. ACSVL1 is expressed primarily in the liver. Inhibition of ACL by ETC-1002-CoA results in decreased cholesterol synthesis in the liver and lowers LDL-C in blood via upregulation of low-density lipoprotein receptors.
12.2. Pharmacodynamics
Administration of bempedoic acid in combination with maximally tolerated statins, with or without other lipid modifying agents, decreases LDL-C, non-high density lipoprotein cholesterol (non-HDL-C), apolipoprotein B (apo B), and total cholesterol (TC) in patients with hyperlipidemia.
Cardiac Electrophysiology
At a dose of 240 mg (1.3 times the approved recommended dose), bempedoic acid does not prolong the QT interval to any clinically relevant extent.
12.3. Pharmacokinetics
Bempedoic acid pharmacokinetic parameters are presented as the mean [standard deviation ± (SD)] unless otherwise specified. The steady-state maximum plasma concentration (Cmax) and area under the curve (AUC) following multiple-dose administration of bempedoic acid at 180 mg/day were 20.6 ± 6.1 µg/mL and 289.0 ± 96.4 µg∙h/mL, respectively. Bempedoic acid steady-state pharmacokinetics were generally linear over a range of >60 mg to 220 mg (approximately 33% to 122% of the recommended dosage of 180 mg daily). There were no time-dependent changes in bempedoic acid pharmacokinetics following repeat administration at the recommended dosage, and bempedoic acid steady-state was achieved after 7 days. The mean accumulation ratio was approximately 2.3-fold.
The steady-state Cmax and AUC of the active metabolite (ESP15228) of bempedoic acid were 2.8 ± 0.9 µg/mL and 51.2 ± 17.2 µg∙h/mL, respectively. ESP15228 likely made a minor contribution to the overall clinical activity of bempedoic acid based on systemic exposure, relative potency, and pharmacokinetic properties.
Absorption
Pharmacokinetic data indicate that bempedoic acid is absorbed with a median time to maximum concentration of 3.5 hours when administered as NEXLETOL 180 mg tablets.
Effect of Food
Concomitant food administration had no effect on the oral bioavailability of bempedoic acid.
Distribution
The bempedoic acid apparent volume of distribution (V/F) was 18 L. Plasma protein binding of bempedoic acid, its glucuronide and its active metabolite, ESP15228, were 99.3%, 98.8% and 99.2%, respectively. Bempedoic acid does not partition into blood cells.
Elimination
The steady-state clearance (CL/F) of bempedoic acid was 11.2 mL/min after once-daily dosing; renal clearance of unchanged bempedoic acid represented less than 2% of total clearance. The mean ± SD half-life for bempedoic acid in humans was 21 ± 11 hours at steady-state.
Metabolism
The primary route of elimination for bempedoic acid is through metabolism of the acyl glucuronide. Bempedoic acid is also reversibly converted to an active metabolite (ESP15228) based on aldo-keto reductase activity observed in vitro from human liver. Mean plasma AUC metabolite/parent drug ratio for ESP15228 following repeat-dose administration was 18% and remained constant over time. Both compounds are converted to inactive glucuronide conjugates in vitro by UGT2B7. Bempedoic acid, ESP15228 and their respective conjugated forms were detected in plasma with bempedoic acid accounting for the majority (46%) of the AUC0-48h and its glucuronide being the next most prevalent (30%). ESP15228 and its glucuronide represented 10% and 11% of the plasma AUC0-48h, respectively.
Excretion
Following single oral administration of 240 mg of bempedoic acid (1.3 times the approved recommended dose), approximately 70% of the total dose (bempedoic acid and its metabolites) was recovered in urine, primarily as the acyl glucuronide conjugate of bempedoic acid, and approximately 30% was recovered in feces. Less than 5% of the administered dose was excreted as unchanged bempedoic acid in feces and urine combined.
Specific Populations
Patients with Renal Impairment
Pharmacokinetics of bempedoic acid was evaluated in a single-dose pharmacokinetic study in subjects with varying degrees of renal function. The mean bempedoic acid AUC in subjects with mild renal impairment (n=8) were 1.5-fold higher compared to those with normal renal function (n=6). Relative to those with normal renal function, mean bempedoic acid AUCs were higher in patients with moderate (n=5) or severe (n=5) renal impairment by 2.3-fold and 2.4-fold, respectively.
A population pharmacokinetic analysis was performed on pooled data from all clinical trials (n=2261) to further evaluate the effects of renal function on the steady-state AUC of bempedoic acid. Compared to patients with normal renal function, the mean bempedoic acid exposures were higher in patients with mild or moderate renal impairment by 1.4-fold (90% CI: 1.3, 1.4) and 1.9-fold (90% CI: 1.7, 2.0), respectively. These differences were not clinically significant. Clinical studies of NEXLETOL did not include patients with severe renal impairment (eGFR <30 mL/min/1.73 m²) or patients with ESRD on dialysis [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
The pharmacokinetics of bempedoic acid and its metabolite (ESP15228) was studied in patients with normal hepatic function or mild or moderate hepatic impairment (Child-Pugh A or B) following a single dose (n=8/group). Compared to patients with normal hepatic function, the bempedoic acid mean Cmax and AUC were decreased by 11% and 22%, respectively, in patients with mild hepatic impairment and by 14% and 16%, respectively, in patients with moderate hepatic impairment. Compared to patients with normal hepatic function, the ESP15228 mean Cmax and AUC were decreased by 13% and 23%, respectively, in patients with mild hepatic impairment and by 24% and 36%, respectively, in patients with moderate hepatic impairment. This is not expected to result in lower efficacy.
Bempedoic acid was not studied in patients with severe hepatic impairment (Child Pugh C) [see Use in Specific Populations (8.7)].
Other Specific Populations
The pharmacokinetics of bempedoic acid were not affected by age, gender, race, or weight.
Drug Interaction Studies
Cytochrome P450 Substrates
In vitro metabolic interaction studies suggest that bempedoic acid, as well as its active metabolite and glucuronide forms are not metabolized by and do not interact with cytochrome P450 enzymes.
Transporter-mediated Drug Interactions
In vitro drug interaction studies suggest bempedoic acid, as well as its active metabolite and glucuronide form, are not substrates of commonly characterized drug transporters with the exception of bempedoic acid glucuronide, which is an OAT3 substrate. Bempedoic acid weakly inhibits OAT3 at high multiples of clinically relevant concentrations, and bempedoic acid and its glucuronide weakly inhibit OATP1B1, and OATP1B3 at clinically relevant concentrations. Bempedoic acid weakly inhibits OAT2 in vitro, which is likely the mechanism responsible for minor elevations in serum creatinine and uric acid [see Adverse Reactions (6.1)].
Probenecid
Administration of bempedoic acid 180 mg with steady-state probenecid resulted in a 1.7- and a 1.2-fold increase in bempedoic acid AUC and Cmax, respectively. AUC and Cmax for bempedoic acid active metabolite (ESP15228) were increased 1.9- and 1.5-fold, respectively. These elevations are not clinically meaningful and do not impact dosing recommendations.
Statins
The pharmacokinetic interactions between bempedoic acid (at systemic exposure relevant to the indicated ASCVD population) and simvastatin 20 mg, atorvastatin 10 mg, pravastatin 40 mg, and rosuvastatin10 mg were evaluated in clinical trials.
Simvastatin: Administration of simvastatin 20 mg with 240 mg of bempedoic acid or 40 mg with 180 mg of bempedoic acid in healthy subjects at steady-state resulted in approximately 2-fold (91% for 20 mg and 96% for 40 mg) and 1.5-fold (54% for 20 mg and 52% for 40 mg) increases in simvastatin acid AUC and Cmax, respectively [See Drug Interactions (7)].
Pravastatin: Administration of pravastatin 40 mg with steady-state bempedoic acid 240 mg in healthy subjects resulted in 99% (2-fold) and 104% (2-fold) increases in pravastatin acid AUC and Cmax, respectively [see Drug Interactions (7)].
Atorvastatin and Rosuvastatin: Elevations of 1.7-fold in AUC of atorvastatin, and rosuvastatin and/or their major metabolites were observed, suggesting a weak interaction. These elevations were generally within the individual statin exposures and do not impact dosing recommendations.
Ezetimibe
Increases in AUC and Cmax for ezetimibe were less than 20% when a single dose of ezetimibe was taken with steady-state bempedoic acid. Total ezetimibe (ezetimibe and its glucuronide form) and ezetimibe glucuronide AUC and Cmax increased approximately 1.6- and 1.8-fold, respectively. These elevations are not clinically meaningful and do not impact dosing recommendations.
Warfarin
In vitro studies indicate that bempedoic acid is not an inhibitor or inducer of CYP2C9. Because warfarin is primarily eliminated through CYP2C9, its pharmacokinetics is not expected to be altered by bempedoic acid.
Other
Bempedoic acid had no effect on the pharmacokinetics of metformin or the oral contraceptive Ortho-Novum 1/35.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Bempedoic acid was negative for mutagenicity in an in vitro Ames assay and negative for clastogenicity in the vitro human lymphocyte chromosome aberration assay. Bempedoic acid was negative in both in vivo mouse micronucleus and in vivo rat bone marrow micronucleus/liver comet assay. In a 2-year rat carcinogenicity study, Wistar rats were given oral doses of bempedoic acid at 3, 10 and 30 mg/kg/day. An increased incidence of liver hepatocellular adenomas and hepatocellular adenomas combined with carcinomas, thyroid gland follicular cell adenoma and follicular cell adenomas combined with carcinomas, and pancreatic islet cell adenomas combined with carcinomas were observed in male rats at the dose of 30 mg/kg/day (exposure equivalent to the maximum recommended human dose (MRHD), based on AUC). In a 2-year mice carcinogenicity study, CD-1 mice were given oral doses of bempedoic acid at 25, 75 and 150 mg/kg/day. Bempedoic acid-related increases in the incidence of liver hepatocellular adenomas, hepatocellular carcinomas and hepatocellular adenomas combined with carcinomas in male mice were observed at 75 and 150 mg/kg/day (exposures equivalent to the MRHD). Observations of liver and thyroid tumors are consistent with PPAR alpha agonism in rodents. The human relevance of pancreatic islet cell tumor findings is unknown.
In fertility and early embryofetal development study in rats, bempedoic acid was given orally to male and female rats at 10, 30 and 60 mg/kg/day. Males were dosed for 28 days prior to mating and females were dosed 14 days prior to mating through gestation day 7. No adverse effects on fertility were observed in females in the absence of maternal toxicity. No effects were observed on male fertility outcomes, but decreases in sperm counts were observed at 60 mg/kg/day (9 times the MRHD).
14. Clinical Studies
The efficacy of NEXLETOL was investigated in two multi-center, randomized, double-blind, placebo-controlled trials that enrolled 3009 adult patients with heterozygous familial hypercholesterolemia or established atherosclerotic cardiovascular disease who were on maximally tolerated statin therapy. Demographics and baseline disease characteristics were balanced between the treatment arms in all trials. In both trials, the maximum LDL-C lowering effects occurred at Week 4. These results were consistent across all subgroups studied in any of the trials, including age, gender, race, ethnicity, region, history of diabetes, baseline LDL-C, body mass index (BMI), HeFH status, and background therapies.
Study 1 (NCT02666664)
Study 1 was a multi-center, randomized, double-blind, placebo-controlled 52-week trial that evaluated safety and efficacy of bempedoic acid in patients with HeFH and/or ASCVD. Efficacy of NEXLETOL was evaluated at Week 12. The trial included 2230 patients randomized 2:1 to receive either NEXLETOL (n=1488) or placebo (n=742) as add-on to a maximally tolerated lipid lowering therapy. Maximally tolerated lipid lowering therapy was defined as a maximally tolerated statin dose alone or in combination with other lipid-lowering therapies. Patients were stratified by presence of HeFH and by baseline statin intensity. Patients on simvastatin 40 mg per day or higher and patients taking PCSK9 inhibitors were excluded from the trial.
Overall, the mean age at baseline was 66 years (range: 24 to 88 years), 61% were ≥65 years old, 27% women, 2% Hispanic, 96% White, 3% were Black, and 1% Asian. Ninety-five percent (95%) of patients had established atherosclerotic cardiovascular disease, and 5% of patients had HeFH. Twenty-nine percent (29%) of patients had diabetes at baseline. The mean baseline LDL-C was 103.2 mg/dL. At the time of randomization, all patients were receiving statin therapy and 50% were receiving high-intensity statin therapy.
The primary efficacy outcome measure of the study was the percent change from baseline to Week 12 in LDL-C. The difference between NEXLETOL and placebo in mean percent change in LDL-C from baseline to Week 12 was -18% (95% CI: -20%, -16%; p<0.001). High-density lipoprotein (HDL) and triglycerides (TG) were examined as exploratory endpoints and were not included in the statistical hierarchy. The difference between NEXLETOL and placebo in mean percent change from baseline to Week 12 was -6% for HDL and median percent change from baseline to Week 12 was +3% for TG. For additional results see Table 2 and Figure 1.
Table 2. Effects of NEXLETOL on Lipid Parameters in Patients with HeFH and/or ASCVD on Maximally Tolerated Statin Therapy (Mean % Change from Baseline to Week 12 in Study 1):
| LDL-C*,† | Non-HDL-C† | apo B† | TC† | |
|---|---|---|---|---|
| NEXLETOL ± Statin ± Other Lipid Lowering Therapies (180 mg/day; n=1488‡) | -17 | -12 | -9 | -10 |
| Placebo (n=742‡) | 2 | 2 | 3 | 1 |
| Mean Difference from Placebo (95% CI) | -18 (-20, -16) | -13 (-15, -12) | -12 (-14, -10) | -11 (-13, -10) |
apo B = apolipoprotein B; CI = confidence interval; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TC = total cholesterol.
Background statin: atorvastatin, simvastatin, pravastatin
* 4 .3% of subjects on NEXLETOL and 2.3% of subjects on placebo had missing LDL-C data at primary endpoint (Week 12). By the end of the trial (Week 52), 8.3% of subjects on NEXLETOL and 7.7% of subjects on placebo had missing LDL-C measurements.
† Percent change from baseline was analyzed using analysis of covariance (ANCOVA), with treatment and randomization strata (HeFH versus ASCVD, and high intensity statin versus other statin) as factors and baseline lipid parameter as a covariate. Missing data for LDL-C, non-HDL-C, TC and apo B were imputed through multiple imputation using a pattern mixture model (PMM) account for treatment adherence.
‡ Number of randomized subjects at baseline.
Study 2 (NCT02991118)
Study 2 was a multi-center, randomized, double-blind, placebo-controlled, 52-week trial in patients with HeFH and/or ASCVD. Efficacy of NEXLETOL was evaluated at Week 12. The trial included 779 patients randomized 2:1 to receive either NEXLETOL (n=522) or placebo (n=257) as add-on to a maximally tolerated lipid lowering therapy. Maximally tolerated lipid lowering therapy was defined as a maximally tolerated statin dose alone or in combination with other lipid-lowering therapies. Patients were stratified by presence of HeFH and baseline statin intensity. Patients on simvastatin 40 mg/day or higher were excluded from the trial.
Overall, the mean age at baseline was 64 years (range: 28 to 91 years), 51% were ≥65 years old, 36% women, 8% Hispanic, 94% White, 5% were Black, and 1% Asian. Ninety-five percent (95%) of patients had established atherosclerotic cardiovascular disease, and 5% of patients had HeFH. Thirty percent (30%) of patients had diabetes at baseline. The mean baseline LDL-C was 120.4 mg/dL. At the time of randomization, 90% of patients were receiving statin therapy, 53% were receiving high-intensity statin therapy, and 0.3% were receiving PCSK9 inhibitors.
The primary efficacy outcome measure of the study was the percent change from baseline to Week 12 in LDL-C. The difference between NEXLETOL and placebo in mean percent change in LDL-C from baseline to Week 12 was -17% (95% CI: -21%, -14%; p<0.001). HDL and TG were exploratory endpoints and not included in the statistical hierarchy. The difference between NEXLETOL and placebo in mean percent change from baseline to Week 12 was -6% for HDL and the median percent change from baseline was -2% for TG. For additional results see Table 3 and Figure 1.
Table 3. Effects of NEXLETOL on Lipid Parameters in Patients with HeFH and/or ASCVD on Maximally Tolerated Statin Therapy (Mean % Change from Baseline to Week 12 in Study 2):
| LDL-C*,† | Non-HDL-C† | apo B† | TC† | |
|---|---|---|---|---|
| NEXLETOL ± Statin ± Other Lipid Lowering Therapies (180 mg/day; n=522‡) | -15 | -11 | -9 | -10 |
| Placebo (n=257‡) | 2 | 2 | 4 | 1 |
| Difference from Placebo (95% CI) | -17 (-21, -14) | -13 (-16, -10) | -13 (-16, -10) | -11 (-14, -9) |
apo B = apolipoprotein B; CI = confidence interval; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; TC = total cholesterol.
Background statin: atorvastatin, simvastatin, rosuvastatin, pravastatin, fluvastatin, pitavastatin, and lovastatin.
* 4.6% of subjects on NEXLETOL and 1.6% of subjects on placebo had missing LDL-C data at primary endpoint (Week 12). By the end of the trial (Week 52), 10.5% of subjects on NEXLETOL and 7.8% of subjects on placebo had missing LDL-C measurements.
† Percent change from baseline was analyzed using analysis of covariance (ANCOVA), with treatment and randomization strata (HeFH versus ASCVD, and high intensity statin versus other statin) as factors and baseline lipid parameter as a covariate. Missing data for LDL-C, non-HDL-C, TC and apo B were imputed through multiple imputation using a pattern mixture model (PMM) account for treatment adherence.
‡ Number of randomized subjects at baseline
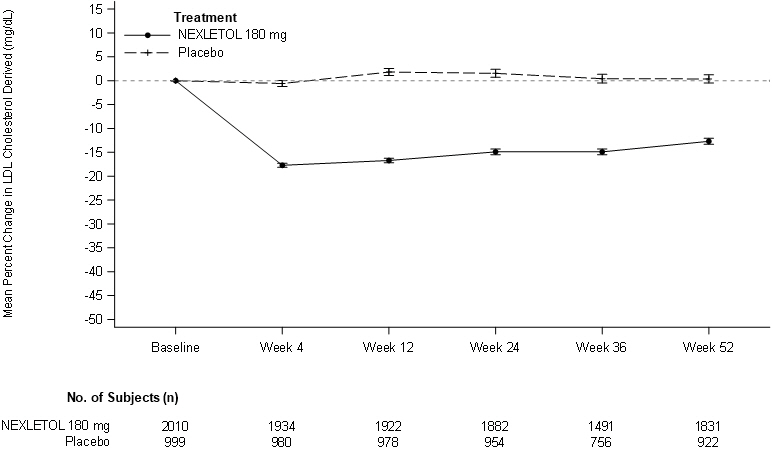
Figure 1. Mean Percent Change from Baseline in LDL-C Over 52 Weeks in Patients with HeFH and/or ASCVD on Maximally Tolerated Statin Treated with NEXLETOL and Placebo (Study 1 and Study 2):
LDL-C derived is calculated from the Friedewald equation: LDL-C = TC – HDL-C – TG/5 in mg/dL.
The error bars represent standard error.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.