NICOTROL Inhaler Ref.[50143] Active ingredients: Nicotine
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
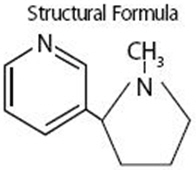
NICOTROL Inhaler (nicotine inhalation system) consists of a mouthpiece and a plastic cartridge delivering 4 mg of nicotine from a porous plug containing 10 mg nicotine. The cartridge is inserted into the mouthpiece prior to use. Nicotine is a tertiary amine composed of a pyridine and a pyrrolidine ring. It is a colorless to pale yellow, freely water-soluble, strongly alkaline, oily, volatile, hygroscopic liquid obtained from the tobacco plant. Nicotine has a characteristic pungent odor and turns brown on exposure to air or light. Of its two stereoisomers, S(-)nicotine is the more active. It is the prevalent form in tobacco, and is the form in the NICOTROL Inhaler. The free alkaloid is absorbed rapidly through skin, mucous membranes, and the respiratory tract.
Chemical Name: S-3-(1-methyl-2-pyrrolidinyl) pyridine
Molecular Formula: C10H14N2
Molecular Weight: 162.23
Ionization Constants: pKa1 = 7.84, pKa2 = 3.04 at 15°C
Octanol-Water Partition Coefficient: 15:1 at pH 7
Nicotine is the active ingredient; inactive components of the product are menthol and a porous plug which are pharmacologically inactive. Nicotine is released when air is inhaled through the Inhaler.
| How Supplied |
|---|
|
NICOTROL INHALER (nicotine inhalation system) is supplied as 168 cartridges each containing 10 mg (4 mg is delivered) nicotine (NDC 0009-5400-01). Each unit consists of 5 mouthpieces, 28 storage trays each containing 6 cartridges and 1 plastic storage case. A patient information leaflet is enclosed with the package. Distributed by Pharmacia & Upjohn Co, Division of Pfizer Inc, NY, NY 10017 |
Drugs
| Drug | Countries | |
|---|---|---|
| NICOTROL | New Zealand, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.