NICOTROL Inhaler Ref.[50143] Active ingredients: Nicotine
Source: FDA, National Drug Code (US) Revision Year: 2021
2. Clinical Pharmacology
Pharmacologic Action
Nicotine, the chief alkaloid in tobacco products, binds stereo-selectively to nicotinic-cholinergic receptors at the autonomic ganglia, in the adrenal medulla, at neuromuscular junctions, and in the brain. Two types of central nervous system effects are believed to be the basis of nicotine’s positively reinforcing properties. A stimulating effect is exerted mainly in the cortex via the locus ceruleus and a reward effect is exerted in the limbic system. At low doses the stimulant effects predominate while at high doses the reward effects predominate. Intermittent intravenous administration of nicotine activates neurohormonal pathways, releasing acetylcholine, norepinephrine, dopamine, serotonin, vasopressin, beta-endorphin, growth hormone, and ACTH.
Pharmacodynamics
The cardiovascular effects of nicotine include peripheral vasoconstriction, tachycardia, and elevated blood pressure. Acute and chronic tolerance to nicotine develops from smoking tobacco or ingesting nicotine preparations. Acute tolerance (a reduction in response for a given dose) develops rapidly (less than 1 hour), but not at the same rate for different physiologic effects (skin temperature, heart rate, subjective effects). Withdrawal symptoms such as cigarette craving can be reduced in most individuals by plasma nicotine levels lower than those from smoking.
Withdrawal from nicotine in addicted individuals can be characterized by craving, nervousness, restlessness, irritability, mood lability, anxiety, drowsiness, sleep disturbances, impaired concentration, increased appetite, minor somatic complaints (headache, myalgia, constipation, fatigue), and weight gain. Nicotine toxicity is characterized by nausea, abdominal pain, vomiting, diarrhea, diaphoresis, flushing, dizziness, disturbed hearing and vision, confusion, weakness, palpitations, altered respiration and hypotension.
Both smoking and nicotine can increase circulating cortisol and catecholamines, and tolerance does not develop to the catecholaminereleasing effects of nicotine. Changes in the response to a concomitantly administered adrenergic agonist or antagonist should be watched for when nicotine intake is altered during NICOTROL Inhaler therapy and/or smoking cessation (See PRECAUTIONS, Drug Interactions).
Pharmacokinetics
Absorption
Most of the nicotine released from the NICOTROL Inhaler is deposited in the mouth. Only a fraction of the dose released, less than 5%, reaches the lower respiratory tract. An intensive inhalation regimen (80 deep inhalations over 20 minutes) releases on the average 4 mg of the nicotine content of each cartridge of which about 2 mg is systemically absorbed. Peak plasma concentrations are typically reached within 15 minutes of the end of inhalation.
Absorption of nicotine through the buccal mucosa is relatively slow and the high and rapid rise followed by the decline in nicotine arterial plasma concentrations seen with cigarette smoking are not achieved with the inhaler. After use of the single inhaler the arterial nicotine concentrations rise slowly to an average of 6 ng/mL in contrast to those of a cigarette, which increase rapidly and reach a mean Cmax of approximately 49 ng/mL within 5 minutes.
The temperature dependency of nicotine release from the NICOTROL Inhaler was studied between 68°F and 104°F in eighteen patients. Average achievable steady state plasma levels after 20 minutes of an intensive inhalation regimen each hour at ambient room temperature are on the order of 23 ng/mL. The corresponding nicotine plasma levels achievable at 86°F and 104°F are on the order of 30 and 34 ng/mL. Nicotine peak plasma concentration (Cmax) at steady-state, after 20 minutes of an intensive inhalation regimen per hour, for 10 hours.
| Cmax (ng/mL) | |||
|---|---|---|---|
| 20°C/68°F | 30°C/86°F | 40°C/104°F | |
| N=18 | N=18 | N=18 | |
| Mean | 22.5 | 29.7 | 34.0 |
| S.D. | 7.7 | 8.3 | 6.9 |
| Min | 11.1 | 17.6 | 24.1 |
| Max | 40.4 | 47.2 | 48.6 |
Ad libitum use of the NICOTROL Inhaler typically produces nicotine plasma levels of 6–8 ng/mL, corresponding to about ⅓ of those achieved with cigarette smoking.
Distribution
The volume of distribution following IV administration of nicotine is approximately 2 to 3 L/kg. Plasma protein binding of nicotine is <5%. Therefore, changes in nicotine binding from use of concomitant drugs or alterations of plasma proteins by disease states would not be expected to have significant effects on nicotine kinetics.
Metabolism
More than 20 metabolites of nicotine have been identified, all of which are less active than the parent compound. The primary urinary metabolites are cotinine (15% of the dose) and trans-3-hydroxycotinine (45% of the dose). Cotinine has a half-life of 15 to 20 hours and concentrations that exceed nicotine by 10-fold. The major site for the metabolism of nicotine is the liver. The kidney and lung are also sites of nicotine metabolism.
Elimination
About 10% of the nicotine absorbed is excreted unchanged in the urine. This may be increased to up to 30% with high urine flow rates and urinary acidification below pH 5. The average plasma clearance is about 1.2 L/min in a healthy adult smoker. The apparent elimination half-life of nicotine is 1 to 2 hours.
Gender Differences
Intersubject variability coefficients of variation (C.V.) for the pharmacokinetic parameters (AUC and Cmax) were approximately 40% and 30% respectively, for males and females. There were no medically significant differences between females and males in the kinetics of NICOTROL Inhaler.
Renal Impairment
Progressive severity of renal impairment is associated with decreased total clearance of nicotine. Nicotine clearance was decreased by 30% on average in subjects with moderate renal impairment and 50% on average in subjects with severe renal impairment.
17.119 Hepatic Impairment
In smokers with liver cirrhosis but only mild impairment of hepatic function (Child-Pugh score 5), the pharmacokinetics of nicotine is unaffected. However, in smokers with moderately impaired liver function (Child-Pugh score 7), total clearance has been reported to be reduced on average by 40–50%. There are no data about the pharmacokinetics of nicotine in smokers with a Child-Pugh score exceeding 7 but these subjects are expected to show similar or greater effects on clearance of nicotine as patients with moderately impaired liver function.
CLINICAL TRIALS
The efficacy of NICOTROL Inhaler therapy as an aid to smoking cessation was demonstrated in two single-center, placebo-controlled, double-blind trials with a total of 445 healthy patients. The number of NICOTROL Inhaler cartridges used was a minimum dose of 4 cartridges/day and a maximum dose of 20 cartridges/day. In both studies, the recommended duration of treatment was 3 months; however, the patients were permitted to continue to use the product for up to 6 months, if they wished. The quit rates are the percentage of all persons initially enrolled who continuously abstained after week 2. NICOTROL Inhaler was more effective than placebo at 6 weeks, 3 months and 6 months. The efficacy is shown in the following table.
| Quit Rates by Treatment (N=445 Patients in 2 Studies) | |||||
|---|---|---|---|---|---|
| Group | Number of Patients | At 6 Weeks | At 3 Months | At 6 Months | At 12 Months* |
| Nicotrol Inhaler | 223 | 44–45% | 31–32% | 20–21% | 11–13% |
| Placebo> | 222 | 14–23% | 8–15% | 6–11% | 5–10% |
* Follow-up, patients not on treatment.
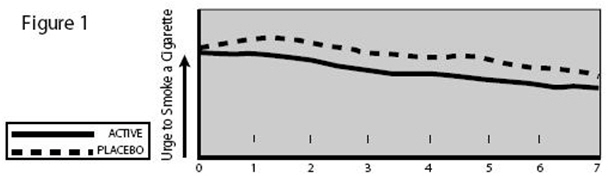
Patients who used NICOTROL Inhaler had a significant reduction in the “urge to smoke”, a major nicotine withdrawal symptom, compared with placebo-treated patients throughout the first week, (see Figure 1).
6.6. Carcinogenesis, Mutagenesis, Impairment of Fertility
Nicotine itself does not appear to be a carcinogen in laboratory animals. However, nicotine and its metabolites increased the incidences of tumors in the cheek pouches of hamsters and forestomach of F344 rats, respectively when given in combination with tumor-initiators. One study, which could not be replicated, suggested that cotinine, the primary metabolite of nicotine, may cause lymphoreticular sarcoma in the large intestine of rats. Neither nicotine nor cotinine was mutagenic in the Ames salmonella test. Nicotine-induced reparable DNA damage in an E. coli test system. Nicotine was shown to be genotoxic in a test system using Chinese hamster ovary cells. In rats and rabbits, implantation can be delayed or inhibited by a reduction in DNA synthesis that appears to be caused by nicotine. Studies have shown a decrease in litter size in rats treated with nicotine during gestation.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.