NIPRIDE RTU Solution for injection Ref.[10893] Active ingredients: Nitroprusside
Source: FDA, National Drug Code (US) Revision Year: 2018
Product description
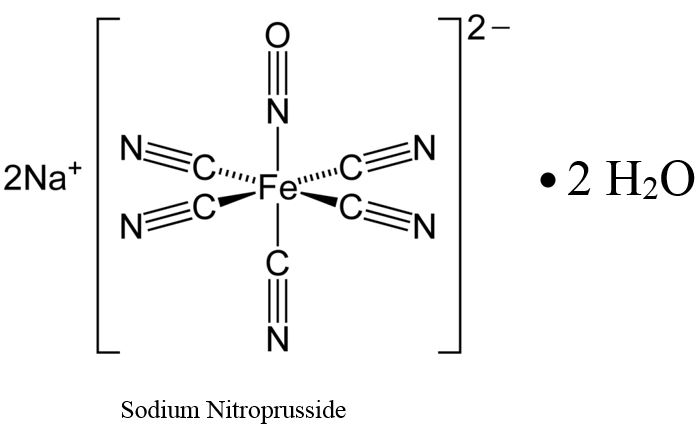
Sodium nitroprusside is disodium pentacyanonitrosylferrate(2-) dihydrate, a hypotensive agent whose structural formula is:
Sodium Nitroprusside has molecular formula Na2[Fe(CN)5NO] • 2H2O and molecular weight of 297.95. Dry sodium nitroprusside is a reddish-brown powder, soluble in water.
Sodium nitroprusside solution is rapidly degraded by trace contaminants, often with resulting color changes [see Dosage and Administration (2.1)].
NIPRIDE RTU is supplied as a sterile, unpreserved, colorless to red-brown solution packaged in a single-use 100-mL vial. Each 100 mL of solution in vial contains 50 mg of sodium nitroprusside (0.5 mg/mL), 900 mg of sodium chloride, USP (9 mg/mL), in sterile water for injection, USP.
NIPRIDE RTU is also supplied as a sterile, unpreserved, colorless to red-brown solution packaged in a single-use 100-mL vial. Each 100 mL of solution in vial contains 20 mg of sodium nitroprusside (0.2 mg/mL), 900 mg of sodium chloride, USP (9 mg/mL), in sterile water for injection, USP.
NIPRIDE RTU is also supplied as a sterile, unpreserved, colorless to red-brown solution packaged in a single-use 50-mL vial. Each 50 mL of solution in vial contains 10 mg of sodium nitroprusside (0.2 mg/mL), 450 mg of sodium chloride, USP (9 mg/mL), in sterile water for injection, USP.
| Dosage Forms and Strengths |
|---|
|
Injection: 50 mg/100 mL of 0.9% sodium chloride (0.5 mg/mL), 20 mg/100 mL of 0.9% sodium chloride (0.2 mg/mL), and 10 mg/50 mL of 0.9% sodium chloride (0.2 mg/mL). NIPRIDE RTU is supplied as a sterile, unpreserved, colorless to red-brown solution available in a single-use vial. |
| How Supplied |
|---|
|
NIPRIDE RTU is supplied in amber-colored, single-dose, 50 mg/100 mL (0.5 mg/mL) Fliptop Vials (NDC 51754-1006-1), 20 mg/100 mL (0.2 mg/mL) Fliptop Vials (NDC 51754-1029-1) and 10 mg/50 mL (0.2 mg/mL) Fliptop Vials (NDC 51754-1018-1). To protect NIPRIDE RTU from light, vial should be stored in its carton until used. |
Drugs
| Drug | Countries | |
|---|---|---|
| NIPRIDE | Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.