NIPRIDE RTU Solution for injection Ref.[10893] Active ingredients: Nitroprusside
Source: FDA, National Drug Code (US) Revision Year: 2018
12.1. Mechanism of Action
Sodium nitroprusside interacts with oxyhemoglobin to produce methemoglobin, cyanide, and nitric oxide (NO). NO then reacts with guanylate cyclase in vascular smooth muscle to produce cGMP that reduces intracellular calcium concentrations resulting in relaxation of vascular smooth muscle and consequent dilatation of peripheral arteries and veins. Other smooth muscle (e.g., uterus, duodenum) is not affected. Sodium nitroprusside is more active on veins than on arteries, but this selectivity is much less marked than that of nitroglycerin. Dilatation of the veins promotes peripheral pooling of blood and decreases venous return to the heart, thereby reducing left ventricular end diastolic pressure and pulmonary capillary wedge pressure (preload). Arteriolar relaxation reduces systemic vascular resistance, systolic arterial pressure, and mean arterial pressure (afterload). Dilatation of the coronary arteries also occurs.
12.2. Pharmacodynamics
In association with the decrease in blood pressure, sodium nitroprusside administered intravenously to hypertensive and normotensive patients produces slight increases in heart rate and a variable effect on cardiac output. In hypertensive patients, moderate doses induce renal vasodilatation roughly proportional to the decrease in systemic blood pressure, so there is no appreciable change in renal blood flow or glomerular filtration rate.
The hypotensive effect of sodium nitroprusside is seen within a minute or two after the start of an adequate infusion, and it dissipates almost as rapidly after an infusion is discontinued. The effect is augmented by ganglionic blocking agents and inhaled anesthetics.
12.3. Pharmacokinetics
Infused sodium nitroprusside is rapidly distributed to a volume that is approximately coextensive with the extracellular space. The drug is cleared by intraerythrocytic reaction with hemoglobin (Hgb), and sodium nitroprusside’s resulting circulatory half-life is about 2 minutes.
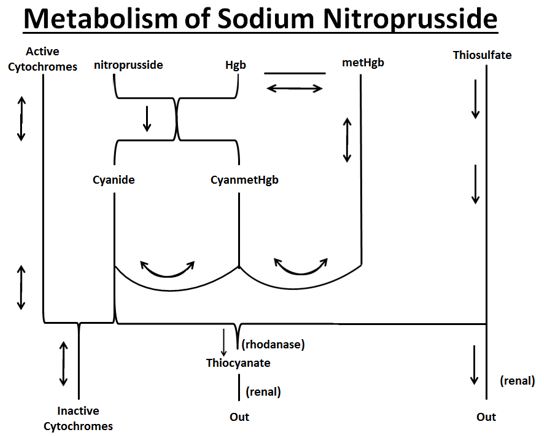
The products of the nitroprusside/hemoglobin reaction are cyanmethemoglobin (cyanmetHgb) and cyanide ion (CN¯).
Metabolism
As shown in the diagram below, the essential features of nitroprusside metabolism are:
- one molecule of sodium nitroprusside is metabolized by combination with hemoglobin to produce one molecule of cyanmethemoglobin and four CN¯ ions;
- methemoglobin, obtained from hemoglobin, can sequester cyanide as cyanmethemoglobin;
- thiosulfate reacts with cyanide to produce thiocyanate;
- thiocyanate is eliminated in the urine;
- cyanide not otherwise removed binds to cytochromes; and
- cyanide is much more toxic than methemoglobin or thiocyanate.
Cyanide ion is normally found in serum; it is derived from dietary substrates and from tobacco smoke. CN¯ levels in packed erythrocytes are typically less than 1 μmol/L (less than 25 mcg/L); levels are roughly doubled in heavy smokers.
At healthy steady state, most people have less than 1% of their hemoglobin in the form of methemoglobin. Nitroprusside metabolism can lead to methemoglobin formation. Relatively large quantities of sodium nitroprusside, however, are required to produce significant methemoglobinemia.
At physiologic methemoglobin levels, the CN¯ binding capacity of packed red cells is a little less than 200 μmol/L (5 mg/L). Cytochrome toxicity is seen at levels only slightly higher, and death has been reported at levels from 300 to 3000 μmol/L (8–80 mg/L). A patient with a normal redcell mass (35 mL/kg) and normal methemoglobin levels can buffer about 175 mcg/kg of CN¯, corresponding to a little less than 500 mcg/kg of infused sodium nitroprusside.
Thiocyanate (SCN¯) is a normal physiological constituent of serum, with normal levels typically in the range of 50-250 μmol/L (3-15 mg/L). Clearance of SCN¯ is primarily renal. In renal failure, the half-life can be doubled or tripled.
When thiosulfate is being supplied only by normal physiologic mechanisms, conversion of CN¯ to SCN¯ generally proceeds at about 1 mcg/kg/min. This rate of CN¯ clearance corresponds to steady-state processing of a sodium nitroprusside infusion of slightly more than 2 mcg/kg/min. CN¯ begins to accumulate when sodium nitroprusside infusions exceed this rate.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies assessing sodium nitroprusside’s carcinogenicity and mutagenicity have not been conducted. Similarly, sodium nitroprusside has not been tested for effects on fertility.
13.2. Animal Toxicology and/or Pharmacology
The acute intravenous mean lethal doses (LD50) of nitroprusside in rabbits, dogs, mice, and rats are 2.8, 5.0, 8.4, and 11.2 mg/kg, respectively.
14. Clinical Studies
Baseline-controlled clinical trials have uniformly shown that sodium nitroprusside has a prompt hypotensive effect, at least initially, in all populations. With increasing rates of infusion, sodium nitroprusside has been able to lower blood pressure without an observed limit of effect.
Clinical trials have also shown that the hypotensive effect of sodium nitroprusside is associated with reduced blood loss in a variety of major surgical procedures.
In patients with acute heart failure and increased peripheral vascular resistance, administration of sodium nitroprusside causes reductions in peripheral resistance, increases in cardiac output, and reductions in left ventricular filling pressure.
Progressive tachyphylaxis to the hypotensive effects of sodium nitroprusside has been reported in several trials and numerous case reports. The mechanism of tachyphylaxis to sodium nitroprusside remains unknown.
Pediatric
The effects of sodium nitroprusside to induce hypotension were evaluated in two trials in pediatric patients less than 17 years of age. In both trials, at least 50% of the patients were pre-pubertal, and about 50% of these pre-pubertal patients were less than 2 years of age, including 4 neonates. The primary efficacy variable was the mean arterial pressure (MAP).
There were 203 pediatric patients in a parallel, dose-ranging study (Study 1). During the 30-minute blinded phase, patients were randomized 1:1:1:1 to receive sodium nitroprusside 0.3, 1, 2, or 3 mcg/kg/min. The infusion rate was increased step-wise to the target dose rate (i.e., ⅓ of the full rate for the first 5 minutes, ⅔ of the full rate for the next 5 minutes, and the full dose rate for the last 20 minutes). If the investigator believed that an increase to the next higher dose rate would be unsafe, the infusion remained at the current rate for the remainder of the blinded infusion. Since there was no placebo group, the change from baseline likely overestimates the true magnitude of blood pressure effect. Nevertheless, MAP decreased 11 to 20 mmHg from baseline across the four doses (Table 1).
There were 63 pediatric patients in a long-term infusion trial (Study 2). During an open-label phase (12 to 24 hours), sodium nitroprusside was started at 0.3 mcg/kg/min and titrated according to the BP response.
Patients were then randomized to placebo or to continuing the same dose of sodium nitroprusside. The average MAP was greater in the control group than in the sodium nitroprusside group for every time point during the blinded withdrawal phase, demonstrating that sodium nitroprusside is effective for at least 12 hours.
In both studies, similar effects on MAP were seen in all age groups.
Table 1. Change from Baseline in MAP (mmHg) After 30 Minutes Double-Blind Infusion (Study 1):
| Dose (mcg/kg/min) | ||||
|---|---|---|---|---|
| Endpoint | 0.3 | 1 | 2 | 3 |
| (N = 50) | (N = 49) | (N = 53) | (N = 51) | |
| Baseline | 76 ± 11 | 77 ± 15 | 74 ± 12 | 76 ± 12 |
| 30 Min | 65 ± 13 | 60 ± 15 | 54 ± 12 | 60 ± 18 |
| Change from | -11 ± 16 | -17 ± 13 | -20 ± 16 | -17 ± 19 |
| Baseline | (-15, -6.5) | (-21, -13) | (-24, -13) | (-22, -11) |
Mean ± SD (95% CI)
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.