NORSPAN Patch Ref.[50647] Active ingredients: Buprenorphine
Source: Medicines and Medical Devices Safety Authority (NZ) Revision Year: 2022 Publisher: Distributed on behalf of Mundipharma New Zealand Limited by: Pharmaco (N.Z.) Ltd, Fisher Crescent, Mt Wellington, Auckland 1060 Ph: (09) 377-3336 Toll Free [Medical Enquiries]: 0800 773 310
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Analgesics, opioids
ATC code: N02AE01
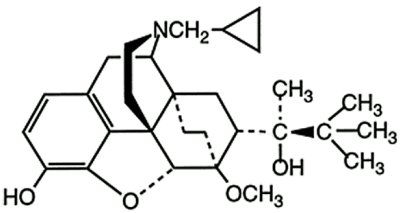
Buprenorphine base (active). CAS Registry Number 52485-79-7.
The structural formula is:
Buprenorphine is a white or almost white powder and is very slightly soluble in water, freely soluble in acetone, soluble in methanol and ether and slightly soluble in cyclohexane. The pKa is 8.5. The chemical name of buprenorphine is (2S)-2-[17-(cyclopropylmethyl)-4, 5α-epoxy-3-hydroxy-6-methoxy-6α, 14-ethano-14α-morphinan-7α-yl]-3, 3-dimethylbutan-2-ol. The molecular weight is 467.6 and the empirical formula is C29H41NO4.
Buprenorphine is a µ-opioid agonist, acting at mu-opioid receptors. The opioid agonist activities of buprenorphine are dose related. Buprenorphine also has antagonistic activity at the kappa-opioid receptor. It is classified as a psychotropic substance under international convention.
Like other opioid agonists, buprenorphine produces dose-related analgesia, however, a ceiling effect to analgesia is well documented. Buprenorphine binds to and dissociates from the mu-receptor slowly, which may account for the prolonged duration of analgesia and, in part, for the limited physical dependence potential observed with the medicine.
Buprenorphine produces similar effects to other opioids on the central nervous, cardiovascular, respiratory and gastrointestinal systems, although the intensity and duration of the effects may vary when compared with other opioids. Opioids may also influence the hypothalamic-pituitary-adrenal or – gonadal axes, including an increase in serum prolactin and decreases in plasma cortisol and testosterone, which can manifest in clinicalsymptoms.
Since kappa-receptor agonist activity is related to psychotomimetic and dysphoric effects, buprenorphine is expected to produce fewer psychotomimetic and dysphoric effects than medicines with kappa-agonist activities.
Like other opioid agonists, buprenorphine may produce increases in cerebrospinal fluid pressure, cause altered mentation, mental clouding or amnesia.
Buprenorphine acts to reduce blood pressure in a manner similar to other opioids. NORSPAN patch application resulted in transient decreases in blood pressure in healthy young and elderly subjects, without clinical adverse events.
Like other opioid analgesics, buprenorphine has a potential of respiratory depression. Respiratory depression is less common than with full mu-agonists, such as morphine, and there appears to be a ceiling effect. However, evidence suggests that buprenorphine is a partial agonist with respect to its respiratory depressant activity. When respiratory depression occurs it appears to have a slower onset and longer duration compared with morphine.
Administration of buprenorphine to persons who are physically dependent on full mu-opioid agonists may precipitate an abstinence syndrome depending on the level of physical dependence, and the timing and dose of buprenorphine.
Like other opioids buprenorphine may cause nausea, vomiting, constipation and an increase in biliary tract pressure. Effects on the immune system were seen with natural opioids like morphine in in vitro and animal studies, although the clinical significance of these is unknown. It is not known whether buprenorphine, a semisynthetic opioid, has immunological effects similar to morphine.
Buprenorphine can cause dose-related miosis and urinary retention in some patients.
Clinical Trials
The safety and efficacy of NORSPAN patch in the management of chronic pain has been studied in 10 clinical trials [1,698 patients treated with NORSPAN patch]. The active and placebo-controlled clinical trials included patients with moderate to severe, chronic pain of osteoarthritis, low back and non-cancer pain requiring opioid analgesia. A single trial examined the safety of three doses of NORSPAN patch given for 72 hours to patients following orthopaedic surgery. No trials have been conducted in patients with cancer related pain.
BUPN.CLIN0001 was a randomised, double-blind, double dummy, parallel, equivalence study comparing the efficacy and tolerability of NORSPAN patch 5, 10 and 20mg applied every seven days with sublingual buprenorphine tablets 200 and 400mcg [Temgesic] in 238 patients with moderate to severe pain due to osteoarthritis [hip and/or knee, 85% >one year]. Patients were titrated to optimum pain control over 21 days, and continued at this level for 28 days. Paracetamol was permitted for breakthrough pain and all usage recorded. The primary efficacy variable was pain intensity recorded during the assessment period [Days three and seven, BS-11 scale - see Table 1]. The Per Protocol mean reductions in pain scores ranged from 2.6 to 3.6 across the three daily rating assessments (morning, midday, evening) and the estimated mean difference between both active treatment arms was minimal [range 0.001 to 0.13]. The 95% confidence intervals for the difference between treatments were within the range -1 to 1, compared with the pre-specified equivalence margins of -1.5 to 1.5. This demonstrated equivalent efficacy. At study completion 70% [40/51] of patients on patch and 75% [42/51] on tablets rated their pain relief as good or very good.
Table 1. Pain intensity scores in study BUPN.CLIN0001:
| Transdermal buprenorphine patches | Sublingual buprenorphine tablets | |
|---|---|---|
| Dose | Titration to optimum pain control over 21 days with same dose continued for up to 28 days | 200 or 400mcg 6-8 hourly |
| Mean baseline pain intensity* | 6.1 | 6.3 |
| Mean pain intensity scores during assessment [Day 7]* | 3.2 | 3.2 |
There was no difference in escape medication usage and the incidence of discontinuation due to lack of efficacy was similar between the two treatment groups [9% Temgesic vs 14% NORSPAN patch]. The most common adverse events reported were those commonly associated with the use of opioids (nausea, vomiting, dizziness, somnolence, headache and constipation).
BP98-1201 was a randomised, double-blind trial comparing the efficacy and safety of NORSPAN patches 5, 10 and 20mg, applied every seven days, with hydrocodone/paracetamol [2.5mg/250mg] tablets four times a day (qid) in 270 patients with chronic moderate to severe back pain [pain intensity ≥5 BS-11 scale], not controlled by non-opioid analgesia alone [ibuprofen 400mg qid]. Patients were titrated to optimum pain control over 21 days, and continued at this level for 35 days. The primary efficacy variables were average pain intensity [BS-11 scale*] and patient satisfaction with medication over Days 21-56+, refer Table 2. The Intent to Treat (ITT) population mean baseline pain intensity was 7.74 (NORSPAN patch group) compared with 7.65, which reduced through Days 21-56 to 5.96 and 6.04, respectively. The difference (and 95% confidence interval) in average pain intensity between the two treatments was -0.08 [-0.06 to 0.44]. The difference between the two treatments in patient global satisfaction was 0.16 [-0.08 to 0.39]. NORSPAN patch was equally effective as hydrocodone/paracetamol tablets in relieving pain and for patient satisfaction.
Table 2. Pain intensity scores in study BP98-1201:
| Transdermal buprenorphine patches | Hydrocodone/paracetamol tablets | |
|---|---|---|
| Dose | Titration to optimum pain control over 21 days, with same dose continued for 35 days | 1 to 3 hydrocodone/paracetamol [2.5mg/250mg] tablets four times daily |
| Mean baseline pain intensity* | 7.74 [7.5 to 8.0] | 7.65 [7.4 to 7.9] |
| Reduction in pain intensity from baseline to end of study* | 1.78 | 1.61 |
| Average pain intensity over Days 21-56* | 5.96 [5.6 to 6.3] | 6.04 [5.7 to 6.4] |
| Patient global satisfaction with medication over Days 21-56+ | 1.53 [1.4 to 1.7] | 1.37 [1.2 to 1.5] |
* Pain intensity was assessed by the BS-11 pain scale, an 11-point scale for rating current pain, where 0 = "no pain" and 10 = "pain as bad as you can imagine".
+ Patient global satisfaction with medication was assessed on a 4-point scale, with the question "Rate the study medication you received for pain".
The majority of adverse events reported were mild or moderate in severity and were typically associated with opioid therapy. Withdrawals due to lack of efficacy was similar for both groups (15% for NORSPAN patch and 14% for hydrocodone/paracetamol). No changes in laboratory values were considered related to treatment, and no clinically important changes were reported for pulse rate, respiratory rate or physical examinations.
5.2. Pharmacokinetic properties
Each NORSPAN patch provides a steady delivery of buprenorphine for up to seven days. Steady state is achieved by day three following the first application. After removal of a NORSPAN patch buprenorphine concentrations initially decline at a rate of approximately 50% in 12 hours. Thereafter, mean elimination half-lives have been reported to be between 30 and 45 hours.
Absorption
Following NORSPAN patch application, buprenorphine diffuses from the patch through the skin. In clinical pharmacology studies, the median time for NORSPAN patch 10 micrograms per hour to deliver detectable buprenorphine concentrations (100 picograms/mL) was approximately 17 hours. The mean bioavailability of buprenorphine from a NORSPAN patch relative to intravenous (IV) dosing is 15% (for all three strengths).
The absorption does not vary significantly across the specified application sites. Mean exposure (AUC) at each of the application sites is within approximately +/- 11% of the mean exposure for the four sites; upper outer arm, upper chest, upper back and the side of the chest.
Accidental oral ingestion: Measurable systemic levels of buprenorphine were demonstrated in dogs given NORSPAN patch by oral administration.
Distribution
Buprenorphine is approximately 96% bound to plasma proteins.
In a study of IV buprenorphine in healthy subjects, the volume of distribution at steady state was 430L, which is indicative of the high lipophilicity of the medicine.
Following IV administration, buprenorphine and its metabolites are secreted into bile, and within several minutes distribute into the cerebrospinal fluid (CSF). CSF concentrations appear to be approximately 15% to 25% of concurrent plasma concentrations.
Biotransformation and Elimination
Buprenorphine metabolism in the skin following NORSPAN patch application is negligible. Buprenorphine is eliminated via hepatic metabolism, with subsequent biliary excretion and renal excretion of soluble metabolites. Hepatic metabolism through CYP3A4 and UGT1A1/1A3 enzymes, results in two primary metabolites, norbuprenorphine and buprenorphine 3-O-glucuronide.
Norbuprenorphine is also glucuronidated prior to elimination. Buprenorphine is also eliminated in the faeces within seven days. Norbuprenorphine is the only known active metabolite of buprenorphine. It has been shown to be a respiratory depressant in rats at concentration at least 50-fold those seen following application of NORSPAN patch 20 micrograms per hour.
In a study in postoperative patients the total clearance of buprenorphine was 55L/h.
Application Site
A study in healthy subjects demonstrated that the pharmacokinetic profile of buprenorphine delivered by NORSPAN patch is similar when applied to the upper outer arm, upper chest, upper back or the side of the chest (midaxillary line, 5th intercostal space).
In a study of healthy subjects applying NORSPAN patch repeatedly to the same site, immediate reapplication caused increased absorption, without clinical adverse events. For this reason, rotation of application sites is recommended (see Section 4.2 Dosage and Administration).
In another study in healthy subjects application of a heating pad directly on the NORSPAN patch caused a transient, 26-55% increase in blood concentrations of buprenorphine. Concentrations returned to normal within five hours after the heat was removed. For this reason, applying heat sources such as hot water bottles, heat pads or electric blankets directly to the NORSPAN patch is not recommended. A heating pad applied to a NORSPAN patch site directly after patch removal did not alter absorption from the skin depot.
5.3. Preclinical safety data
Systemic toxicity and dermal toxicity
In single- and repeat-dose toxicity studies in rats, rabbits, guinea pigs, dogs, and minipigs, NORSPAN caused minimal or no adverse systemic events, whereas skin irritation was observed in all species examined. Toxicological data available did not indicate a sensitising potential of the additives of the transdermal patches
Genotoxicity and carcinogenicity
A standard battery of genotoxicity texts indicated that buprenorphine is non-genotoxic. In long-term studies in rats and mice there was no evidence of any carcinogenic potential relevant for humans.
Reproductive and developmental toxicity
No effect on fertility or general reproductive performance was observed in rats treated with buprenorphine. No embryofetal toxicity effects were observed in rats or rabbits. In a rat pre- and postnatal development toxicity study with buprenorphine there was pup mortality and decreased pup body weight at maternal doses that produced a reduction in food consumption and clinical signs.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.