OLESTYR Oral suspension Ref.[49692] Active ingredients: Colestyramine
Source: Health Products and Food Branch (CA) Revision Year: 2022
Clinical pharmacology
10.1 Mechanism of Action
Cholestyramine is a quaternary ammonium anion exchange resin with a polystyrene polymer skeleton. As the chloride salt, it binds bile acids both in vitro and in vivo, exchanging chloride for bile acid. Cholesterol is probably the sole precursor of bile acids. During normal digestion, bile acids are secreted into the intestines. A major portion of the bile acids is absorbed from the intestinal tract and returned to the liver via the enterohepatic circulation. Only very small amounts of bile acids are found in normal serum.
Cholestyramine resin absorbs and combines with the bile acids in the intestine to form an insoluble complex which is excreted in the feces. This results in a partial removal of bile acids from the enterohepatic circulation by preventing their absorption.
The increased fecal loss of bile acids due to cholestyramine resin administration leads to an increased oxidation of cholesterol to bile acids, a decrease in beta lipoprotein or low-density lipoprotein plasma levels and a decrease in serum cholesterol levels. Although in humans cholestyramine resin produces an increase in hepatic synthesis of cholesterol, plasma cholesterol levels fall.
10.2 Pharmacodynamics
Animal Pharmacology
Binding of Bile Acids
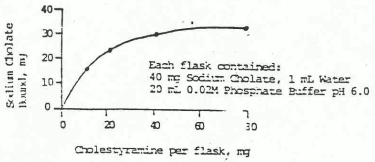
Since cholestyramine is an anion exchange resin, the chloride anion attached the quaternary ammonium groups of the resin can be replaced by other anions - usually those with a greater affinity for the resin than chloride. Bile acids are strongly bound by the resin as shown in in vitro studies.
Figure 1. Binding of Sodium Chelate by Cholestyramine In Vitro:
A 3-fold increase in fecal bile acid excretion after 10 days was reported. This effect continued during nine weeks of administration of a normal diet, containing 2% cholestyramine, fed to male albino rats of 130-140 grams.
Binding of Drugs
Since cholestyramine is an anion exchange resin, it has a strong affinity for acidic materials. It may also absorb neutral or, less likely, basic materials to some extent.
Eleven drugs have been studied in vivo and in vitro for possible binding with cholestyramine:
| Basic | Neutral | Acidic |
|---|---|---|
| Chlorpheniramine maleates | Digoxin | Acetylsalicylic Acid |
| Dextromethorphan | Chlorothiazide | |
| Dihydrocodeinone bitartrates | Phenobarbital | |
| Quinidine sulfate | Phenylbutazone | |
| Tetracycline | ||
| Warfarin |
The basic and neutral drugs were not bound, or bound only slightly, by cholestyramine in vitro. Those which were weakly bound were very easily washed from the resin with buffer at various pH levels.
Acetylsalicylic Acid, although an acidic drug, had much less affinity and was more easily eluted form cholestyramine than cholic acid. In support of these in vitro results, the blood level of salicylic acid was only moderately depressed in the first half hour following the concomitant oral administration of acetylsalicylic acid, at a dose of 4.65 mg/kg and cholestyramine, at a dose of 71.5 mg/kg, to rats. After two hours, blood salicylate levels were not affected by the resin.
Similar in vivo and in vitro results were observed with phenobarbital and tetracycline.
The absorption of phenylbutazone may be delayed (but not decreased) when taken with cholestyramine, as suggested by studies in the rat.
No significant effects on chlorothiazide absorption or excretion were observed in dogs given chlorothiazide 30 minutes before the administration of cholestyramine.
In rats, the anticoagulant activity of a large single dose of warfarin was unaffected by the administration of cholestyramine, whether warfarin was given 30 minutes before or simultaneously within the resin. Plasma warfarin levels were lower when the two drugs were given together.
Fat Absorption
In a study with male weanling rats, the administration of 5% cholestyramine decreased the absorption of medium chain triglycerides by 3%, whereas absorption of the other dietary fats was more markedly affected. Five percent cholestyramine decreased net absorption of coconut oil by 15%, the highly unsaturated vegetable oils by 19 to 40%, olive oil by 40% and butter and lard by 47 and 55%, respectively.
Fat-Soluble Vitamins A and K Absorption
The inclusion of 1 or 2% cholestyramine in rations containing 5 20% fat and minimal levels of vitamin A led to decreased liver stores of vitamin A in young rats. No overt evidence of a nutritional deficiency of this essential vitamin was observed. Rates of weight gain and efficiency of caloric utilization were unaffected at the lower levels of dietary fat intake.
In studies of 1 to 8-day old chicks fed minimal or adequate amounts of menadione (a synthetic analog of vitamin K), the addition of 2% cholestyramine to the diet had no significant effect on prothrombin time after 2 or 4 weeks.
Human Pharmacology
Binding of Bile Acids
Cholestyramine is a quaternary ammonium anion exchange resin with a polystyrene polymer skeleton. As the chloride salt, it binds bile acids both in vitro and in vivo, exchanging chloride for bile acid. When the resin is administered to certain animals used for experimental purposes and to man, it sequesters bile acids in the gut, preventing their reabsorption and thereby promoting their excretion in the feces.
Fat Absorption
Clinical investigators induced gross steatorrhea in two healthy subjects by the administration of a large daily dose (30 g) of cholestyramine for 11-17 days. Fecal fat excretion increased by factors of 4 and 5, respectively, returning promptly to pre-treatment values when cholestyramine was withdrawn.
Studies in 5 healthy subjects, maintained on regular diet and given radioactive labelled triolein, before and during administration of 30 g/day of cholestyramine, demonstrated that there was a depression in the level of blood radioactivity over the 8-hour sampling period, and significant increase in fecal radioactivity during the 48-hour period of cholestyramine administration.
In contrast, in 7 subjects maintained on regular diet and given radioactive labeled oleic acid, there was no significant difference in radioactivity of blood and feces between control and experimental periods.
The investigators suggested that the binding of bile acids by cholestyramine prevents their participation in the hydrolytic digestion of dietary triglycerides. This, in turn, leads to the steatorrhea induced by large doses of cholestyramine.
Studies in a limited number of patients with partial biliary obstruction have demonstrated that serum bile acids, phospholipids, triglycerides, cholesterol and total lipids may be lowered during treatment with cholestyramine, although another investigator reported significant decreases in serum triglyceride levels in only 4 of 15 patients.
Fat-Soluble Vitamins A and K Absorption
Using four healthy young adult subjects, investigatorsreported that when 8 grams of cholestyramine was ingested simultaneously with a normal meal with 250,000 U.S.P. Units of vitamin A acetate, during a 9-hour postprandial period, the plasma vitamin A levels were significantly reduced (below the values obtained with the control meal). The 4 grams addition of cholestyramine had no significant effect.
Clinical Studies
1. Hypercholesterolemia
In proper dosage, cholestyramine usually leads to a significant reduction (15% or more) in serum cholesterol levels. This result from the increased fecal loss of bile acids bound to the resin, and the compensatory formation of additional bile acids from cholesterol. The lowering of serum cholesterol levels has been observed both in subjects with "normal" cholesterol levels (100 250 mg/100 mL) as well as in patients with elevated values.
In a careful, long term metabolic study of 10 patients with hypercholesterolemia, investigators reported that over periods of 12 months for 7 patients and 6 months for 3 patients with varying dosage levels of cholestyramine (12 24 g/day) the decrease in cholesterol ranged from 15 to 76% of an average of pre-treatment values. The mean decrease was 43%. Another investigatorreported studies on 17 patients with varying degrees of hypercholesterolemia, for most of whom he prescribed 4 8 grams cholestyramine daily. (Two patients received 12 g/day). Significant cholesterol reductions occurred in many of these patients with an average reduction of 23.5%.
One investigatoremphasized the importance of carefully determining the etiology of the hypercholesterolemia that is to be treated. He finds that patients who are truly idiopathic, and not basically hypertriglyceridemic, respond to cholestyramine with significant lowering of serum cholesterol. This investigator observed 13 patients with idiopathic hypercholesterolemia who experienced an average cholesterol reduction of 26% with dosage of 8 to 16 g daily, for a period of one month to two years.
The National Institutes of Health have concluded a 10-year randomized double blind placebocontrolled study, in men, at 12 lipid research clinics on the effect of lowering plasma cholesterol on the risk of coronary heart disease defined as CHD death and/or non fatal myocardial infarction. The 3,806 participants who took part in this study were preponderantly college or high school educated whites. Their mean age was 47.8 years. Upon entering the study, all participants had a plasma cholesterol level of 265 mg/dL or greater and an LDL-C level of 190 mg/dL or greater. Participants with coronary heart disease or conditions associated with secondary hyperlipoproteinemia were excluded from the study. The effect of Total C on incidence of CHD is illustrated in Table 2.
Table 2. Cholesterol Lowering and Coronary Heart Disease:
| N | Mean Total-C* | No. of CHD Cases** | |
|---|---|---|---|
| Cholestyramine Group | 1,906 | 251 | 155 |
| Placebo Group | 1,900 | 276 | 187 |
* Average of annual posttreatment levels for participants attending clinic. TOTAL-C indicates plasma total cholesterol.
** Definite non-fatal myocardial infarction or CHD death.
Plasma cholesterol was lowered by a combination of a modest cholesterol-lowering diet and cholestyramine. The dose response relationship between the amount of cholestyramine ingested daily, the lowering of total plasma cholesterol, and the reduction in CHD risk is summarized in Table 3.
Table 3. Relation of Reduction of Cholesterol to Reduction in Coronary Heart Disease Risk:
| Dose of Cholestyramine | Package Count | Patient Population | Total Cholesterol Lowering | Reduction in CHD Risk |
|---|---|---|---|---|
| 0-8 g | 0-2 | 439 | 4.4% | 10.9% |
| 8-20 g | 2-5 | 496 | 11.5% | 20.1% |
| 20-24 g | 5-6 | 965 | 19.0% | 39.3% |
2. Partial Biliary Obstruction
Bile acids are formed in the liver from cholesterol and excreted via the bile into the intestine. Here they are involved in the digestive processes, emulsifying the fats and fatty materials present in ingested foods. A large proportion of the bile acids is reabsorbed and returned via the portal circulation to the liver.
Very small amounts of bile acids are found in normal sera. When the normal secretion of bile is partially blocked, however, serum concentrations may increase 10 to 20-fold or more. When this occurs an intractable pruritus often intervenes. This pruritus may be so severe that some patients become extremely depressed.
Several recent reports show that administration of cholestyramine reduced serum bile acids and relieved pruritus in such patients. Withholding the resin for a few days led to a return of pruritus and increased serum bile acid levels.
These observations support the hypothesis of a causal relationship between high serum bile acid concentrations and the pruritus of jaundice. The lag periods of several days between administration of the resin and relief of itching, and between withholding cholestyramine and the return of itching, suggest that the causative factor may not be bile acid in the serum, but that which accumulated in the skin or adjacent tissues.
Increased fecal bile acid excretion after cholestyramine administration to man has been consistently observed. One investigator reported an increase in fecal bile acid from 54 to 500 mg/day, in a patient, following the ingestion of cholestyramine.
Investigators observed an increase in fecal bile acids from a mean of 81 mg/day during a 10-day control period to 364 mg/day during 54 days of cholestyramine therapy (dosage 1.7-6.6 g/day).
Other investigatorsreported that four patients with pruritus associated with partial biliary obstruction had an average serum bile acid concentration of 25 mcg/mL. During treatment with cholestyramine, the itching was relieved, and serum bile acids averaged only 6 mcg/mL.
Abundant data in human studies demonstrate conclusively that an important effect of cholestyramine is to increase fecal bile acid excretion and reduce serum bile acids.
3. Diarrhea in Post-Ileal Resection Patients
Investigatorsreported that on fifteen patients with persistent diarrhea of more than one year's duration following ileal resection, 13 patients had a 50% reduction in stool frequency and 14 had an improvement in consistency on an average dose of 5.4 g of cholestyramine per day. Urgency, perianal soreness, and flatus also decreased in most cases.
Other investigators observed that the stool frequency decreased in 11 patients when cholestyramine was added to the diet and was further decreased when Portagen was substituted for part of the dietary fat.
10.3 Pharmacokinetics
Pharmacokinetic studies have not been carried out with OLESTYR as cholestyramine is not absorbed from the GI tract.
Microbiology
No microbiological information is required for this drug product.
Toxicology
16 NON-CLINICAL TOXICOLOGY
General Toxicology
Oral chronic toxicity studies lasting for one year were conducted in rats and in dogs. Dosages of cholestyramine greatly in excess of those used in man exhibited no toxic manifestations and caused no observable histological changes in either species.
In these studies, the rats were fed 0.5, 1 or 2 g of cholestyramine per kg of body wei ght each day. The beagle dogs received 5, 10, or 20 g daily. No adverse effects on weight or other gross clinical signs of toxicity were observed in either species.
In the dogs, periodic measurements of total red cells, hematocrit, hemoglobin, sedimentati on rate and differential leucocyte counts were made. Serum glucose, BUN, carbonate, chloride, sodium, potassium and pH measurements were not remarkable; nor were urinary tests for protein, sugar, pH, chloride, sodium and potassium. Similar measurements were made on the rats during the year as far as samples of blood and urine could be obtained. No abnormal values attributable to cholestyramine administration were observed.
Carcinogenicity / Genotoxicity
Studies were conducted in rats in which cholestyramine resin was used as a tool to investigate the role of various intestinal factors (e.g.,fat, bile salts, GI flora). The incidence of intestinal tumors, induced by potent carcinogens, was observed to be greater in cholestyramine resin treated rats, than in control rats.
This observation was not evident in all studies conducted in rats, as results from one study indicated a statistically insignificant increase in tumor incidence whereas a more recent study did not demonstrate any presence of tumors following ingestion of cholestyramine. The relevance of this laboratory observation from studies in rats to the clinical use of OLESTYR is not known.
Reproductive and Developmental Toxicology
Three successive litters of rats were bred, whelped, and weaned from dams and sires fed 2 grams of cholestyramine per kg of body weight daily, beginning 60 days before the initial breeding and continuing through all periods of pregnancy, lactation, and intervening rest. There was no evidence of gross toxicity among the parent animals. Reproductive performance was normal, and pregnancy and lactation proceeded smoothly. Fetal development was normal. No gross teratogenic effects were observed to be associated with cholestyramine administration. Pup growth rates, and body weights at birth and weaning were normal.
Occasional oral, nasal and ocular porphyrin discharges were observed both in control and treated animals. One treated animal exhibited corneal opacity, and another, a growth on the right side, toward the end of the 37-week study. Neither was considered unusual nor cholestyramine-induced. Other anomalous changes common in rats included hydronephrosis and diaphragmatic hernia in a few animals, observed in proportionately equivalent numbers among the control and experimental groups. No gross pathology due to cholestyramine was observed in any parental animals, and no evaluation of possible skeletal anomalies was made in the offspring.
Extra care was required to assure the nutritional adequacy of the ration for the cholestyramine-fed animals, as evidenced by decreased pup mortality when the standard diet was supplemented with vitamins.
Under the conditions of these studies, when cholestyramine was fed at levels 10 times the usual human dose, the only adverse effects were nutritional, due to sequestration of one or more essential vitamins by the agent.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.