ONUREG Film-coated tablet Ref.[28040] Active ingredients: Azacitidine
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: Bristol-Myers Squibb Pharma EEIG, Plaza 254, Blanchardstown Corporate Park 2, Dublin 15, D15 T867, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, antimetabolites, pyrimidine analogues
ATC code: L01BC07
Mechanism of action
Azacitidine is a DNA methyltransferase inhibitor and epigenetic modifier. Azacitidine is incorporated into DNA and RNA following cellular uptake and enzymatic biotransformation to nucleotide triphosphates. Incorporation of azacitidine into the DNA of AML cells, modified epigenetic pathways through the inhibition of DNA methyltransferases, and reduction of DNA methylation. This led to alteration of gene expression, including re-expression of genes regulating tumour suppression, immune pathways, cell cycle, and cell differentiation. Incorporation of azacitidine into the RNA of AML cells, inhibited RNA methyltransferase, reduced RNA methylation, decreased RNA stability, and decreased protein synthesis.
Clinical efficacy and safety
The efficacy and safety of Onureg was studied in a multi-centre, placebo-controlled, Phase 3 study QUAZAR AML-001 (CC-486-AML-001) with a double-blind, randomised, parallel-group design which evaluated Onureg versus placebo as maintenance therapy in AML patients. Patients were enrolled with de novo AML, AML secondary to prior diagnosis of myelodysplastic syndromes (MDS), or chronic myelomonocytic leukaemia (CMML); the patients were aged ≥55 years, and had achieved first complete remission (CR) or complete remission with incomplete blood count recovery (CRi) within 4 months (+/- 7 days) after intensive induction chemotherapy with or without consolidation therapy. Patients were not eligible for HSCT at the time of randomisation, which included patients who did not have a transplant donor, or who chose not to proceed to HSCT.
Patients in both treatment arms received best supportive care as deemed necessary by the investigator. Best supportive care included, but was not limited to, treatment with red blood cell (RBC) transfusions, platelet transfusions, use of erythropoiesis stimulating agent, antibiotic, antiviral and/or antifungal therapy, GCSF, anti-emetic therapy, and nutritional support.
Patients who achieved a CR/CRi after completion of intensive induction therapy with or without consolidation were administered Onureg 300 mg (N=236) or placebo (N=233) once daily on Days 1 through 14 of each 28-day cycle. In the event of disease relapse (5% to 15% blasts in peripheral blood or bone marrow), the dose schedule was extended to 21 days of repeated 28-day treatment cycles per medical discretion. Treatment continued until disease progression (more than 15% blasts were observed in peripheral blood or bone marrow) or until unacceptable toxicity.
A total of 472 patients were randomised 1:1 between Onureg and placebo treatment arms. Baseline demographic and disease characteristics for the AML patient population were balanced between treatment arms as shown in Table 3. The median treatment duration was 11.6 months (range: 0.5 to 74.3 months) for the Onureg arm versus 5.7 months (range: 0.7 to 68.5 months) for the placebo arm. A total of 51 patients (21%) receiving Onureg and 40 patients (17%) receiving placebo extended their dose schedule to 300 mg daily for 21 days due to AML disease relapse.
Of the 469 patients in the Phase 3 study who received treatment, 61% (285/469) were 65 years of age or older and 11% (51/469) were 75 years of age or older. No overall differences in safety or efficacy of Onureg were observed between these patients and younger patients.
Table 3. Baseline demographics and disease-related characteristics in study CC-486-AML-001:
| Parameter | Onureg (N=238) | Placebo (N=234) |
|---|---|---|
| Age (years) | ||
| Median (min, max) | 68.0 (55, 86) | 68.0 (55, 82) |
| Age category, n (%) | ||
| <65 years | 66 (27.7) | 68 (29.1) |
| ≥65 years to <75 years | 144 (60.5) | 142 (60.7) |
| ≥75 years | 28 (11.8) | 24 (10.3) |
| Sex, n (%) | ||
| Male | 118 (49.6) | 127 (54.3) |
| Female | 120 (50.4) | 107 (45.7) |
| Race, n (%) | ||
| White | 216 (90.8) | 197 (84.2) |
| Black or African American | 2 (0.8) | 6 (2.6) |
| Asian | 6 (2.5) | 20 (8.5) |
| Other | 12 (5.0) | 11 (4.7) |
| Not collected or reported | 2 (0.8) | 0 (0) |
| ECOG performance status, n (%) | ||
| 0 | 116 (48.7) | 111 (47.4) |
| 1 | 101 (42.4) | 106 (45.3) |
| 2 | 21 (8.8) | 15 (6.4) |
| 3 | 0 (0) | 2 (0.9) |
| Cytogenetic risk status at diagnosis, n (%) | ||
| Intermediate risk1 | 203 (85.3) | 203 (86.6) |
| Poor risk2 | 35 (14.7) | 31 (13.2) |
| Initial AML classification, n (%) | ||
| AML with recurrent genetic abnormalities | 39 (16.4) | 46 (19.7) |
| AML with myelodysplasia-related changes | 49 (20.6) | 42 (17.9) |
| Therapy related myeloid neoplasms | 2 (0.8) | 0 (0) |
| AML not otherwise specified | 148 (62.2) | 145 (62.0) |
| Missing | 0 (0) | 1 (0.4) |
| Type of AML, n (%) | ||
| Primary (de novo) | 213 (89.5) | 216 (92.3) |
| Secondary | 25 (10.5) | 18 (7.7) |
| MRD status at randomisation3, n (%) | ||
| Negative | 133 (55.9) | 111 (47.4) |
| Positive | 103 (43.3) | 116 (49.6) |
| Missing | 2 (0.8) | 7 (3.0) |
AML=Acute myelogenous leukemia; MDS=Myelodysplastic syndrome; CMML=Chronic myelomonocytic Leukemia; ECOG=Eastern cooperative oncology group; CR=Morphologic complete remission; CRi=Morphologic CR with incomplete blood count recovery.
1 Intermediate risk was defined as normal cytogenetics +8, t(9;11), or other undefined.
2 Poor risk was defined as complex (≥3 abnormalities): 5; 5q; 7; 7q; 11q23 - non t(9;11); inv(3); t(3;3); t(6;9); or t(9;22). Source for Intermediate and Poor Risk: National comprehensive cancer network clinical practice guidelines in oncology for AML.
3 MRD status in bone marrow was measured during screening period by flow cytometric assay at a sensitivity level of 0.1%.
Most patients received consolidation therapy after induction therapy in both the Onureg (78%) and placebo (82%) treatment arms; more than 90% of these patients in each treatment arm received 1 or 2 cycles of consolidation therapy after induction therapy (Table 4).
Table 4. Consolidation therapy in study CC-486-AML-001:
| Parameter | Onureg (N=238) | Placebo (N=234) |
|---|---|---|
| Received consolidation therapy following induction | ||
| Yes, n (%) | 186 (78.2) | 192 (82.1) |
| 1 Cycle, n (%) | 110 (46.2) | 102 (43.6) |
| 2 Cycles, n (%) | 70 (29.4) | 77 (32.9) |
| 3 Cycles, n (%) | 6 (2.5) | 13 (5.6) |
| No, n (%) | 52 (21.8) | 42 (17.9) |
| CR/CRi status at randomisation | ||
| CR, n (%) | 183 (76.9) | 177 (75.6) |
| CRi, n (%) | 50 (21.0) | 44 (18.8) |
| Not in CR/CRia, n (%) | 5 (2.1) | 11 (4.7) |
| Missing, n (%) | 0 (0) | 2 (0.9) |
CR=Complete remission; CRi=Morphologic CR with incomplete blood count recovery.
a These patients had baseline bone marrow of less than 5% blasts and both ANC <1 x 109 and platelets <100 x 109.
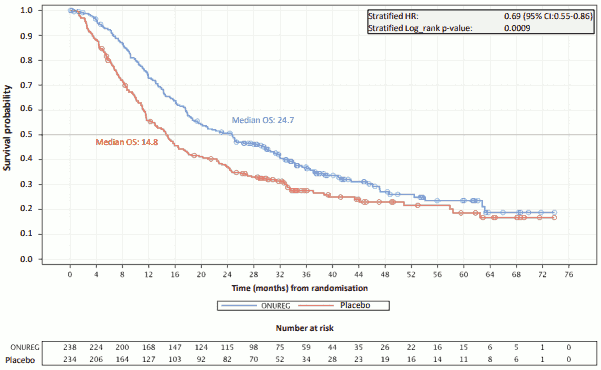
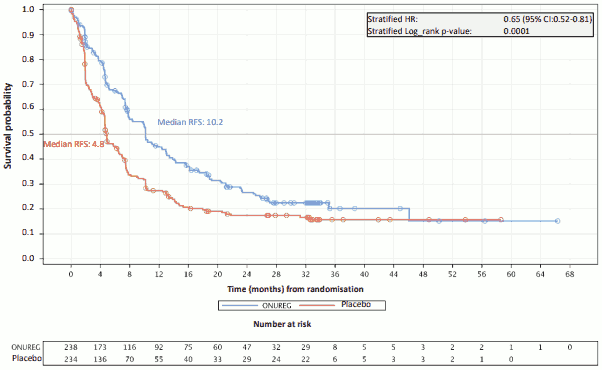
The efficacy of Onureg in adult patients with AML was established based on overall survival (OS) and relapse-free survival (RFS).
The efficacy results are summarised in the Table 5.
Table 5: CC-486-AML-001 efficacy results (ITT Population):
| Endpoints | Onureg (N=238) | Placebo (N=234) |
|---|---|---|
| Overall survival | ||
| OS events, n (%) | 158 (66.4) | 171 (73.1) |
| Median OS, months (95% CI) | 24.7 (18.7, 30.5) | 14.8 (11.7, 17.6) |
| Hazard ratio (95% CI) p-value | 0.69 (0.55, 0.86) 0.0009 | |
| Relapse-free survival | ||
| Events, n (%) | 164 (68.9) | 181 (77.4) |
| Median RFS, months (95% CI) | 10.2 (7.9, 12.9) | 4.8 (4.6, 6.4) |
| Hazard ratio (95% CI) p-value | 0.65 (0.52, 0.81) 0.0001 | |
| Time to relapse | ||
| Relapsed, n (%) | 154 (64.7) | 179 (76.5) |
| Median time to relapse, months (95% CI) | 10.2 (8.3, 13.4) | 4.9 (4.6, 6.4) |
| Time to discontinuation from treatment | ||
| Treatment discontinued, n (%) | 193 (81.1) | 208 (88.9) |
| Median time to treatment discontinuation, months (95% CI) | 11.4 (9.8, 13.6) | 6.1 (5.1, 7.4) |
| Treatment discontinued - disease relapse, n (%) | 143 (60.1) | 180 (76.9) |
CI=Confidence interval.
Prespecified subgroup analyses of OS and RFS showed a consistent treatment effect for Onureg across demographic and disease-related subgroups including baseline cytogenetic risk, the number of prior consolidation cycles received, and CR/CRi status.
The Kaplan-Meier curves display the OS (see Figure 1) and RFS (see Figure 2) results.
Figure 1. Kaplan-Meier curve for overall survival: Onureg versus placebo (ITT Population):
Figure 2: Kaplan-Meier curve for relapse free survival: Onureg versus placebo (ITT Population):
In patients who had their dose schedule extended to 300 mg for 21 days due to disease relapse, the median OS (22.8 months for Onureg and 14.6 months for placebo) and median RFS (7.4 months for Onureg and 4.6 months for placebo) were comparable to the overall study results.
Onureg demonstrated a favorable treatment effect for OS compared with placebo in both minimal residual disease (MRD)-positive and MRD-negative patients. The treatment effect for OS was more pronounced in MRD-positive patients (HR=0.69; 95% CI: 0.51, 0.93) than in MRD-negative patients (HR=0.81; 95% CI: 0.59, 1.12).
Health related quality of life (HRQoL)
HRQoL was assessed using the Functional assessment of chronic illness therapy-fatigue scale (FACIT - fatigue scale) and the Five dimensions three levels (EQ-5D-3L) health utility index and visual analogue scale (VAS). At baseline, patients had a low level of fatigue and good level of HRQoL that were generally comparable to those of the general population of similar age. This level of HRQoL was maintained over time with Onureg, as compared to baseline, as well as to placebo. Both the time to definitive deterioration and the proportion of patients experiencing clinically meaningful deterioration was found to be similar between those receiving Onureg and placebo. Overall, the findings demonstrate that HRQoL was similar between Onureg treatment and placebo arms, with no clinically meaningful deterioration over time.
5.2. Pharmacokinetic properties
Absorption
Exposure was generally linear with dose-proportional increases in systemic exposure; high intersubject variability was observed. The geometric mean (coefficient of variation [%CV]) Cmax and AUC values after oral administration of a 300 mg single dose were 145.1 ng/mL (63.7) and 241.6 ng h/mL (64.5), respectively. Multiple dosing at the recommended dose regimen did not result in drug accumulation.
Absorption of azacitidine was rapid, with a median Tmax of 1 hour post dose. Mean oral bioavailability relative to subcutaneous (SC) administration was approximately 11%.
Effect of food
The impact of food on the exposure of Onureg was minimal. Therefore, Onureg can be administered with or without food.
Distribution
After oral administration, the geometric mean apparent volume of distribution was 12.6 L/kg for a 70 kg person. The plasma protein binding of azacitidine was 6 to 12%.
Biotransformation
Based on in vitro data, azacitidine metabolism does not appear to be mediated by cytochrome P450 isoenzymes (CYPs). Azacitidine undergoes spontaneous hydrolysis and deamination mediated by cytidine deaminase.
Elimination
The geometric mean apparent clearance was 1242 L/hour and the geometric mean half-life was approximately 0.5 hours. Following intravenous administration of 14C azacitidine to 5 cancer patients, the cumulative urinary excretion was 85% of the radioactive dose. Faecal excretion accounted for <1% of administered radioactivity over 3 days. Mean excretion of radioactivity in urine following subcutaneous administration of 14C-azacitidine was 50%. The amount of unchanged azacitidine recovered in urine relative to dose was <2% following either subcutaneous (SC) or oral administration. Faecal excretion has not been measured following oral administration.
Pharmacodynamic effects
The epigenetic regulatory effect of azacitidine on DNA global methylation reduction in the blood was sustained with prolonged exposure of 300 mg daily administered for 14 or 21 days of a 28-day cycle in myeloid cancers including AML patients from a Phase ½ study. A positive correlation was observed between azacitidine plasma exposure and the pharmacodynamic effect of reduction in global DNA methylation in blood.
Special populations
Elderly
In a population pharmacokinetics (PK) analysis from 286 AML patients, age (46 to 93 years) did not have clinically meaningful effects on the PK of Onureg. Therefore, dose modification for Onureg is not required, regardless of patient age.
Hepatic impairment
No formal studies have been conducted in patients with hepatic impairment. Hepatic impairment is unlikely to affect the PK to a clinically relevant extent since azacitidine undergoes spontaneous hydrolysis and deamination mediated by cytidine deaminase. A population PK analysis determined that AST (8 to 155 U/L), ALT (5 to 185 U/L) and mild hepatic impairment (BIL ≤ ULN and AST > ULN, or BIL 1 to 1.5 × ULN and any AST) did not have clinically meaningful effects on the PK of azacitidine. The effects of moderate to severe hepatic impairment (BIL >1.5 × ULN and any AST) on the PK of azacitidine is unknown.
Renal impairment
In patients with cancer, the PK of azacitidine in 6 patients with normal renal function (CLcr >80 mL/min) and 6 patients with severe renal impairment (CLcr <30 mL/min) were compared following daily subcutaneous dosing (Days 1 through 5) at 75 mg/m²/day. Severe renal impairment increased azacitidine exposure by approximately 70% after single and 41% after multiple subcutaneous administrations. This increase in exposure was not correlated with an increase in adverse events.
A population PK analysis following a 300 mg dose of Onureg determined that patients with mild (CLcr: ≥60 to <90 mL/min), moderate (CLcr: ≥30 to <60 mL/min), and severe (CLcr: <30 mL/min) renal impairment had 19%, 25%, and 38% increases in azacitidine plasma AUC, respectively. The effect of severe renal impairment on Onureg was similar to the above referenced clinical renal impairment study with injectable azacitidine (~40% increase in AUC). The exposure of azacitidine (AUC) is approximately 75% lower after oral administration relative to the exposure achieved following SC administration; therefore, an increase in exposure of approximately 40% following oral administration is still considered safe and tolerable. Thus, no dose adjustment of Onureg is recommended in patients with mild, moderate, or severe renal impairment.
Race / ethnicity
The effects of race/ethnicity on the PK of Onureg is unknown.
5.3. Preclinical safety data
In a 14-day oral toxicity study in dogs, mortality occurred at doses of 8 and 16 mg/m²/day. The maximum tolerated dose (MTD) was 4 mg/m²/day. At 1 or all doses, pancytopenia correlated with bone marrow hypoplasia, lymphoid depletion, gland/lumen dilation and single cell necrosis in mucosal crypts of small and large intestines and/or centrilobular hepatocellular vacuolation were observed. At the MTD, these findings were partially or completely resolved after 3 weeks. Following parenteral azacitidine administrations at comparable dose ranges, mortality and similar target organ toxicities were observed in rodents, dogs and monkeys. Non-clinical data from repeat-dose toxicity studies with azacitidine revealed no special hazard for humans.
Azacitidine induces both gene mutations and chromosomal aberrations in bacterial and mammalian cell systems in vitro. The potential carcinogenicity of azacitidine was evaluated in mice and rats. Azacitidine induced tumours of the haematopoietic system in female mice, when administered intraperitoneally 3 times per week for 52 weeks. An increased incidence of tumours in the lymphoreticular system, lung, mammary gland, and skin was seen in mice treated with azacitidine administered intraperitoneally for 50 weeks. A tumorigenicity study in rats revealed an increased incidence of testicular tumours.
Early embryotoxicity studies in mice revealed a 44% frequency of intrauterine embryonal death (increased resorption) after a single intraperitoneal injection of azacitidine during organogenesis. Developmental abnormalities in the brain have been detected in mice given azacitidine on or before closure of the hard palate. In rats, azacitidine caused no adverse reactions when given pre-implantation, but it was clearly embryotoxic when given during organogenesis.
Foetal abnormalities during organogenesis in rats included: Central nervous system (CNS) anomalies (exencephaly/encephalocele), limb anomalies (micromelia, club foot, syndactyly, oligodactyly) and others (microphthalmia, micrognathia, gastroschisis, oedema, and rib abnormalities).
Administration of azacitidine to male mice prior to mating with untreated female mice resulted in decreased fertility and loss of offspring during subsequent embryonic and postnatal development. Treatment of male rats resulted in decreased weight of the testes and epididymides, decreased sperm counts, decreased pregnancy rates, an increase in abnormal embryos and increased loss of embryos in mated females (see section 4.6).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.