ORGOVYX Film-coated tablet Ref.[27984] Active ingredients: Relugolix
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
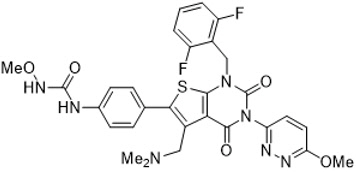
Relugolix is a nonpeptide small molecule, GnRH receptor antagonist. The chemical name is N-(4-{1-[(2,6-difluorophenyl)methyl]-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl}phenyl)-N'-methoxyurea.
The molecular weight is 623.63 daltons and the molecular formula is C29H27F2N7O5S. The structural formula is:
Relugolix is a white to off-white to slightly yellow solid with a solubility of 0.04 mg per mL in water at 25°C.
ORGOVYX is provided as film-coated tablets for oral administration. Each tablet contains 120 mg of relugolix. The inactive ingredients are mannitol, sodium starch glycolate, hydroxypropyl cellulose, magnesium stearate, hypromellose, titanium dioxide, ferric oxide red, and carnauba wax.
| Dosage Forms and Strengths |
|---|
|
Tablets: 120 mg, light red, almond-shaped, film-coated, and debossed with "R" on one side and "120" on the other side. |
| How Supplied |
|---|
|
The 120 mg tablets are film-coated, light red, almond shaped, and debossed with "R" on one side and "120" on the other side and are supplied in two configurations, bottles and blister packs. Each bottle (NDC 72974-120-01) contains 30 tablets and a desiccant and is closed with a child-resistant induction seal cap. The blister cards contain nine tablets packaged in a carton (NDC 72974-120-02). Each ORGOVYX tablet contains 120 mg of relugolix. Manufactured by Bushu Pharmaceuticals, Ltd, Kawagoe, Saitama, Japan Manufactured for Myovant Sciences, Inc., Brisbane, CA 94005 |
Drugs
| Drug | Countries | |
|---|---|---|
| ORGOVYX | Austria, Estonia, Spain, France, Croatia, Ireland, Italy, Lithuania, Romania, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.