ORGOVYX Film-coated tablet Ref.[27984] Active ingredients: Relugolix
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
Relugolix is a nonpeptide GnRH receptor antagonist that competitively binds to pituitary GnRH receptors, thereby, reducing the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and consequently testosterone.
12.2. Pharmacodynamics
Pituitary and Gonadal Hormones
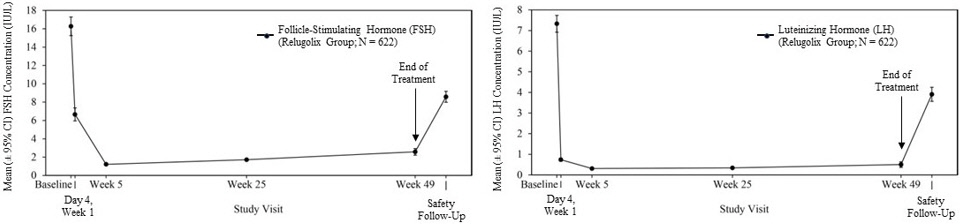
Relugolix reduced LH, FSH (Figure 1), and testosterone concentrations after oral administration of the recommended loading dose of 360 mg and a 120 mg dose once daily.
Out of 622 patients, 56% had testosterone concentrations at castrate levels (<50 ng/dL) by the first sampling timepoint at Day 4, and 97% maintained castrate levels of testosterone through 48 weeks. In a substudy of 137 patients who did not receive subsequent androgen deprivation therapy for at least 90 days after discontinuation of relugolix, the cumulative incidence rate of achieving testosterone concentrations above the lower limit of the normal range (>280 ng/dL) or baseline at 90 days was 55% [see Clinical Studies (14)].
Figure 1. Mean (± 95% CI) Follicle-Stimulating Hormone and Luteinizing Hormone Concentrations over Time in HERO:
Cardiac Electrophysiology
In a randomized, double-blind, placebo- and positive-controlled (open-label moxifloxacin), parallel-group thorough QT/QTc study, no increase in mean QTc interval >10 ms was identified after administration of single 60 or 360 mg doses of relugolix (0.2 or 1 times the recommended loading dose, respectively).
12.3. Pharmacokinetics
After administration of single doses ranging from 60 mg to 360 mg (0.17 to 1 times the recommended loading dose), the AUC from time zero extrapolated to infinity (AUC0-inf) and the maximum observed plasma concentration (Cmax) of relugolix increase approximately proportionally with dose. After administration of multiple 20 mg to 180 mg doses of relugolix once daily (0.17 to 1.5 times the recommended once daily dose), the AUCtau of relugolix increases approximately proportionally with dose and the Cmax increases greater than proportionally to dose. After administration of a single 360 mg loading dose in patients, the mean (± standard deviation [± SD]) of AUC0-24 and Cmax of relugolix were 985 (± 742) ng.hr/mL and 215 (± 184) ng/mL, respectively. After administration of a 120 mg dose once daily in patients, the mean (± SD) of AUC0-24 and Cmax of relugolix at steady-state were 407 (± 168) ng.hr/mL and 70 (± 65) ng/mL, respectively. The accumulation of relugolix upon once daily administration is approximately 2-fold.
Absorption
Relugolix is a substrate for intestinal P-gp. The mean (CV%) absolute bioavailability of relugolix is approximately 12% (62%). The median (range) Tmax of relugolix is 2.25 hours (0.5 to 5.0 hours).
Effect of Food
No clinically meaningful differences in the pharmacokinetics of relugolix were observed following consumption of a high-calorie, high-fat meal (approximately 800 to 1000 calories with 500, 220, and 124 from fat, carbohydrate, and protein, respectively).
Distribution
Plasma protein binding of relugolix is 68 to 71%, primarily to albumin and to a lesser extent to α 1-acid glycoprotein. The mean blood-to-plasma ratio is 0.78.
Elimination
The mean effective half-life of relugolix is 25 hours and the mean (CV%) terminal elimination half-life is 60.8 (11%) hours. The mean (CV%) total clearance of relugolix is 29.4 (15%) L/h and the renal clearance is 8 L/h.
Metabolism
Relugolix is metabolized primarily by CYP3A and to a lesser extent by CYP2C8 in vitro.
Excretion
After oral administration of a single 80-mg radiolabeled dose of relugolix, approximately 81% of the radioactivity was recovered in feces (4.2% as unchanged) and 4.1% in urine (2.2% as unchanged).
Specific Populations
No clinically meaningful differences in the pharmacokinetics of relugolix were observed based on age (45 to 91 years), race/ethnicity (Asian [19%], White [71%], Black/African American [6%]), body weight (41 to 193 kg), mild to severe renal impairment (creatinine clearance [CLcr] 15 to 89 mL/min, as estimated by the Cockcroft-Gault equation), or mild to moderate hepatic impairment (Child-Pugh A or B). The effect of end-stage renal disease with or without hemodialysis or severe hepatic impairment (Child-Pugh C) on the pharmacokinetics of relugolix has not been evaluated.
Drug Interactions Studies
Clinical Studies
Combined P-gp and Moderate CYP3A Inhibitor: Co-administration with erythromycin (P-gp and moderate CYP3A inhibitor) increased the AUC and Cmax of relugolix by 6.2-fold.
Combined P-gp and Strong CYP3A Inducer: Co-administration with rifampin (P-gp and strong CYP3A inducer) decreased the AUC and Cmax of relugolix by 55% and 23%, respectively.
Other Drugs: No clinically significant differences in the pharmacokinetics of relugolix were observed when co-administered with voriconazole (strong CYP3A inhibitor), atorvastatin, enzalutamide, or acid-reducing agents. No clinically significant differences in the pharmacokinetics of midazolam (sensitive CYP3A substrate) or rosuvastatin (BCRP substrate) were observed upon co-administration with relugolix.
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Relugolix is a substrate of CYP3A and CYP2C8. Relugolix is not an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4. Relugolix is an inducer of CYP3A and CYP2B6, but not an inducer of CYP1A2.
Transporter Systems: Relugolix is a substrate of P-gp, but not a substrate of BCRP. Relugolix is an inhibitor of BCRP and P-gp, but not an inhibitor of OATP1B1, OATP1B3, OAT1, OAT3, OCT2, MATE1, MATE2-K, or BSEP.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in mice at oral relugolix doses up to 100 mg/kg/day and in rats at doses up to 600 mg/kg/day. Relugolix was not carcinogenic in mice or rats at exposures up to approximately 75 or 224 times, respectively, the human exposure at the recommended dose of 120 mg daily based on AUC.
Relugolix was not mutagenic in the in vitro bacterial reverse mutation (Ames) assay or clastogenic in the in vitro chromosomal aberration assay in Chinese hamster lung cells or the in vivo rat bone marrow micronucleus assay.
In human GnRH-receptor knock-in male mice, oral administration of relugolix decreased prostate and seminal vesicle weights at doses ≥3 mg/kg twice daily for 28 days. The effects of relugolix were reversible, except for testis weight, which did not fully recover within 28 days after drug withdrawal. In a 39-week repeat-dose toxicity study in monkeys, there were no significant effects on male reproductive organs at oral relugolix doses up to 50 mg/kg/day (approximately 53 times the human exposure at the recommended dose of 120 mg daily based on AUC).
13.2. Animal Toxicology and/or Pharmacology
Phospholipidosis (intracellular phospholipid accumulation) was observed in multiple organs and tissues (e.g., liver, pancreas, spleen, kidney, lymph nodes, lung, bone marrow, gastrointestinal tract or testes) after repeated oral administration of relugolix in rats and monkeys. In a rat 26-week toxicity study, phospholipidosis was observed at doses ≥100 mg/kg (approximately 18 times the human exposure at the recommended dose based on AUC). In a monkey 39-week toxicity study, this effect was observed at doses ≥1.5 mg/kg (approximately 0.6 times the human exposure at the recommended dose based on AUC) and demonstrated evidence of reversibility after cessation of treatment. The significance of this finding in humans is unknown.
14. Clinical Studies
HERO Study
The safety and efficacy of ORGOVYX was evaluated in HERO (NCT03085095), a randomized, open label study in men with advanced prostate cancer requiring at least 1 year of androgen deprivation therapy and defined as biochemical (PSA) or clinical relapse following local primary intervention, newly diagnosed castration-sensitive metastatic disease, or advanced localized disease.
A total of 934 patients were randomized to receive ORGOVYX or leuprolide in a 2:1 ratio for 48 weeks:
- ORGOVYX at a loading dose of 360 mg on the first day followed by daily doses of 120 mg orally
- Leuprolide acetate 22.5 mg injection (or 11.25 mg in Japan and Taiwan) subcutaneously every 3 months. Leuprolide acetate 11.25 mg is a dosage regimen that is not recommended for this indication in the US.
Serum testosterone concentrations were measured at screening; on Days 1, 4, 8, 15, and 29 in the first month; then monthly until the end of the study.
The population (N=930) across both treatment groups had a median age of 71 years (range 47 to 97 years). The ethnic/racial distribution was 68% White, 21% Asian, 4.9% Black, and 5% other. Disease stage was distributed as follows: 32% metastatic (M1), 31% locally advanced (T3/4 NX M0 or any T N1 M0), 28% localized (T1 or T2 N0 M0), and 10% not classifiable. The median testosterone concentration at baseline across the treatment groups was 408 ng/dL.
The major efficacy outcome measure was medical castration rate defined as achieving and maintaining serum testosterone suppression to castrate levels (< 50 ng/dL) by Day 29 through 48 weeks of treatment. Other endpoints included castration rates on Day 4 and 15 and castration rates with testosterone <20 ng/dL at Day 15.
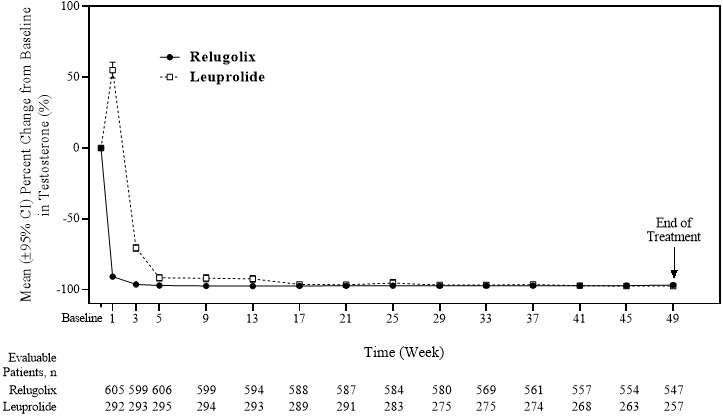
The efficacy results are shown in Table 3 and the timecourse of percent change from baseline in testosterone suppression by ORGOVYX and leuprolide during the 48 week treatment period are shown in Figure 2.
Table 3. Medical Castration Rates (Testosterone Concentrations <50 ng/dL) from Day 29 through Week 48 in HERO:
| ORGOVYX 360/120 mg (N=622)* | Leuprolide Acetate 22.5 or 11.25 mg† (N=308)* | |
|---|---|---|
| Castration Rate (95% CI)‡ | 96.7% (94.9%, 97.9%) | 88.8% (84.6%, 91.8%) |
* Two patients in each arm did not receive the study treatment and were not included.
† 11.25 mg is a dosage regimen that is not recommended for this indication in the US. The castration rate of the subgroup of patients receiving 22.5 mg leuprolide (n=264) was 88.0% (95% CI: 83.4%, 91.4%).
‡ Kaplan-Meier estimates within group.
Figure 2. Mean (95% CI) Percent Change from Baseline in Testosterone Concentrations from Baseline to Week 49 by Treatment Group in HERO:
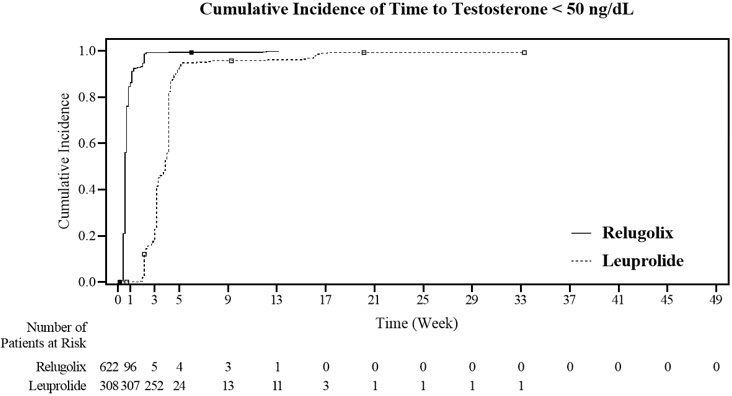
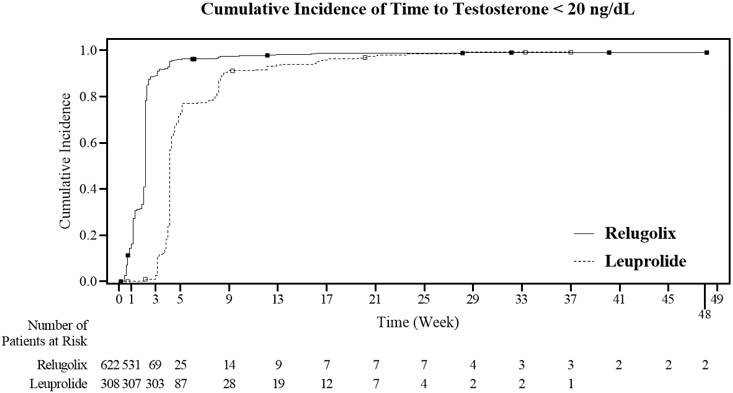
The percentages of patients who attained the medical castration levels of testosterone <50 ng/dL and <20 ng/dL within the first 29 days of treatment are summarized in Table 4 and the cumulative incidences of time to testosterone <50 ng/dL or <20 ng/dL are shown in Figure 3.
Table 4. Percentage of Patients Attaining Testosterone Decreases within the First 29 Days in HERO*:
| Testosterone <50 ng/dL | Testosterone <20 ng/dL | |||
|---|---|---|---|---|
| ORGOVYX (N=622) | Leuprolide Acetate (N=308) | ORGOVYX (N=622) | Leuprolide Acetate (N=308) | |
| Day 4 | 56% | 0% | 7% | 0% |
| Day 8 | 91% | 0% | 27% | 0% |
| Day 15 | 99% | 12% | 78% | 1% |
| Day 29 | 99% | 82% | 95% | 57% |
Figure 3. Cumulative Incidence of Time to Testosterone <50 ng/dL and <20 ng/dL in HERO:
In the clinical trial, PSA levels were monitored and were lowered on average by 65% two weeks after administration of ORGOVYX, 83% after 4 weeks, 92% after 3 months and remained suppressed throughout the 48 weeks of treatment. These PSA results should be interpreted with caution because of the heterogeneity of the patient population studied. No evidence has shown that the rapidity of PSA decline is related to a clinical benefit.
A substudy was conducted in 137 patients who did not receive subsequent androgen deprivation therapy for at least 90 days after discontinuation of ORGOVYX. Based on Kaplan-Meier analyses, 55% of patients achieved testosterone levels above the lower limit of the normal range (>280 ng/dL) or baseline at 90 days after discontinuation of ORGOVYX.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.