ORLADEYO Hard capsule Ref.[28003] Active ingredients: Berotralstat
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: BioCryst Ireland Limited, Block 4, Harcourt Centre, Harcourt Road, DUBLIN 2, D02HW77, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other haematological agents, drugs used in hereditary angioedema
ATC code: B06AC06
Mechanism of action
Berotralstat is an inhibitor of plasma kallikrein. Plasma kallikrein is a serine protease that cleaves high-molecular-weight-kininogen (HMWK), releasing bradykinin, a potent vasodilator that increases vascular permeability. In patients with HAE due to C1-INH deficiency or dysfunction, normal regulation of plasma kallikrein activity is impaired, which leads to uncontrolled increases in plasma kallikrein activity and bradykinin release, resulting in HAE attacks consisting of swelling (angioedema).
Cardiac electrophysiology
At the steady state Cmax of berotralstat at the recommended dose of 150 mg once daily, the mean corrected QT interval increased by 3.4 msec (90% upper CI bound of 6.8 msec), which is below the 10 msec threshold for concern. At a supratherapeutic dose of 450 mg once daily, steady state exposures were 4-fold higher than at the recommended 150 mg dose, and the corrected QT interval increased by a mean of 21.9 msec.
Clinical efficacy and safety
Efficacy of berotralstat was studied in a multicentre, randomised, double-blind, placebo-controlled, parallel-group study NCT 03485911.
Study NCT 03485911
This study included 120 patients (114 adults and 6 children 12 years and over) with type I or II HAE who experienced at least two investigator-confirmed attacks within the first 8 weeks of the run-in period and took at least one dose of study treatment. Nine patients were aged ≥65 years. Patients were randomised into 1 of 3 parallel treatment arms, stratified by baseline attack rate, in a 1:1:1 ratio (berotralstat 110 mg, berotralstat 150 mg or placebo by oral administration once daily, with food) for the 24-week treatment period.
A total of 81 patients received at least one dose of berotralstat in the 24-week treatment period. Overall, 66% of patients were female and 93% of patients were Caucasian with a mean age of 41.6 years. A history of laryngeal angioedema attacks was reported in 74% of patients and 75% reported prior use of long-term prophylaxis. The median attack rate during the prospective run-in period (baseline attack rate) was 2.9 per month. Of patients enrolled, 70% had a baseline attack rate of ≥2 attacks per month.
Patients discontinued other prophylactic HAE medicinal products prior to entering the study; however, all patients were allowed to use rescue medicinal products for treatment of breakthrough HAE attacks. In berotralstat-treated patients, 51.4% of breakthrough attacks were treated with C1-INH (see section 4.4). Concomitant use of C1-INH and berotralstat did not result in any identifiable adverse reactions.
Orladeyo 150 mg produced a statistically significant and clinically meaningful reduction in the rate of HAE attacks compared to placebo through 24 weeks in the primary endpoint Intent-to-Treat (ITT) population as shown in Table 2. The percent reduction in HAE attack rate was greater with Orladeyo 150 mg compared to placebo, regardless of attack rate during the run-in period.
Table 2. Reduction in HAE attack rate in the berotralstat 150 mg ITT population:
| Outcome | Berotralstat 150 mg (n=40) | Placebo (n=40a) | ||
|---|---|---|---|---|
| Rate per 28 days | Percent reduction from placebo (95% CI) | p-value | Rate per 28 days | |
| HAE attack rate | 1.31 | 44.2% (23.0, 59.5) | <0.001 | 2.35 |
a One patient in the ITT analysis was randomised to placebo but was not treated.
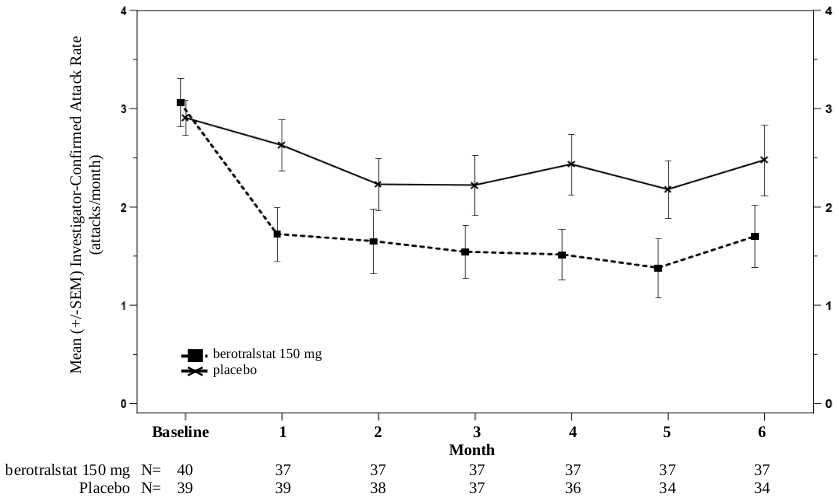
Reduction in attack rates was sustained through 24 weeks, as shown in Figure 1.
Figure 1. HAE attack rate per month through 24 weeks treatment with berotralstat 150 mg (n=40) or placebo (n=40):
SEM: standard error of the mean
Of patients receiving 150 mg berotralstat, 58% had a ≥50% reduction in their HAE attack rates compared to baseline versus 25% of placebo patients.
Orladeyo 150 mg reduced the rate of HAE attacks requiring treatment with standard of care acute attack treatments by 49.2% (95% CI: 25.5%, 65.4%) compared to placebo (rate per 28 days: 1.04 vs. 2.05).
Health-related quality of life
Patients receiving berotralstat 150 mg experienced an improvement in Angioedema Quality of Life Questionnaire (AE-QoL) total score and domain scores (functioning, fatigue/mood, fear/shame and nutrition) compared to the placebo group as shown in Table 3. A reduction of 6 points is considered a clinically meaningful improvement. The largest improvement was observed in the functioning score.
Table 3. Change in AE-QoL score* - berotralstat compared to placebo at week 24:
| LS mean change (SE) from baseline at week 24 | LS mean difference from placebo (95% CI) | ||
|---|---|---|---|
| Berotralstat 150 mg | Placebo | ||
| AE-QoL total score | -14.6 (2.6) | -9.7 (2.6) | -4.90 (-12.23, 2.43) |
| Functioning score | -19.5 (3.4) | -10.4 (3.4) | -9.10 (-18.58, 0.38) |
| Fatigue/Mood score | -11.3 (3.2) | -9.2 (3.3) | -2.16 (-11.35, 7.03) |
| Fear/Shame score | -15.4 (3.2) | -10.5 (3.3) | -4.96 (-14.05, 4.13) |
| Nutrition score | -8.8 (3.0) | -6.1 (3.1) | -2.68 (-11.27, 5.92) |
AE-QoL=Angioedema Quality of Life Questionnaire; CI=confidence interval; LS=least squares; SE=standard error
* Lower scores indicate improved quality of life (lower impairment)
Paediatric population
The safety and effectiveness of Orladeyo were evaluated in 28 adolescent patients aged 12 to <18 years across both studies. The safety profile and attack rate on study were similar to those observed in adults.
The safety and efficacy of berotralstat in paediatric patients under 12 years have not been established.
The European Medicines Agency has deferred the obligation to submit the results of studies with Orladeyo in one or more subsets of the paediatric population in the treatment of hereditary angioedema for the prevention of attacks in patients with hereditary angioedema (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Absorption
Following oral administration of berotralstat 150 mg once daily, Cmax and area under the curve over the dosing interval (AUCtau) are 158 ng/mL (range: 110 to 234 ng/mL) and 2770 ng*h/mL (range: 1880 to 3790 ng*h/mL), respectively. The pharmacokinetics of berotralstat in patients with HAE are similar to those of healthy people.
Berotralstat exposure (Cmax and AUC) increases greater than proportionally with dose and steady state is reached by days 6 to 12.
Food effect
No differences in the Cmax and AUC of berotralstat were observed following administration with a high-fat meal. However the median tmax was delayed by 3 hours, from 2 hours (fasted) to 5 hours (fed, range: 1 to 8 hours). Berotralstat is to be administered with food to minimise gastrointestinal adverse events.
Distribution
Plasma protein binding is approximately 99%. After a single dose of radiolabelled berotralstat 300 mg, the blood to plasma ratio was approximately 0.92. At steady state, the geometric mean (%CV) Vd/F was 3123 L (40%) for berotralstat 150 mg once daily.
Biotransformation
Berotralstat is metabolised by CYP2D6 and by CYP3A4 with low turnover in vitro. After a single oral radiolabelled berotralstat 300 mg dose, berotralstat represented 34% of the total plasma radioactivity, with 8 metabolites, each accounting for between 1.8 and 7.8% of the total radioactivity. Structures for 5 of the 8 metabolites are known. It is unknown whether any metabolites are pharmacologically active.
Berotralstat 150 mg once daily is a moderate inhibitor of CYP2D6 and CYP3A4, and a weak inhibitor of CYP2C9. Berotralstat is not an inhibitor of CYP2C19. Berotralstat at double the recommended dose is a weak inhibitor of P-gp and is not an inhibitor of BCRP.
Elimination
After a single dose of 150 mg, the median half-life of berotralstat was approximately 93 hours (range: 39 to 152 hours).
After a single oral radiolabelled berotralstat 300 mg dose, approximately 9% was excreted in urine (3.4% unchanged; range 1.8 to 4.7%) and 79% was excreted in faeces. Additional analyses indicated approximately 50% of the fraction recovered in the faeces was unchanged berotralstat.
Special populations
Population pharmacokinetic analyses showed that age, gender and race did not meaningfully influence the pharmacokinetics of berotralstat. Body weight was identified as a covariate describing the variability of clearance and volume of distribution, resulting in higher exposure (AUC and Cmax) in patients weighing less. However, this difference is not considered to be clinically relevant and no dose adjustments are recommended for any of these demographics.
Paediatric population
Based on population pharmacokinetic analyses that included paediatric patients 12 to <18 years and weighing at least 40 kg, exposure at steady state following oral administration of berotralstat 150 mg once daily was slightly higher (29% higher) than adult exposure, with an estimated geometric mean (CV%) AUCtau of 2515 (38.6) ng*h/mL. However, this difference is not considered to be clinically relevant, and no dose adjustments are recommended in paediatric patients 12 to <18 years of age weighing 40 kg or more.
Renal impairment
The pharmacokinetics of a single 200 mg oral dose of berotralstat were studied in patients with severe renal impairment (eGFR less than 30 mL/min). When compared to a concurrent cohort with normal renal function (eGFR greater than 90 mL/min); Cmax was increased by 39%, while no difference was observed in AUC. No dose adjustment is required for patients with mild or moderate renal impairment. Patients with severe renal impairment may be at risk of prolonged QT. It is preferable to avoid the use of berotralstat in these patients.
The pharmacokinetics of berotralstat in patients with kidney failure requiring haemodialysis has not been studied. Given the high plasma protein binding of berotralstat, it is unlikely to be cleared by haemodialysis.
Hepatic impairment
The pharmacokinetics of a single 150 mg oral dose of berotralstat were studied in patients with mild, moderate and severe hepatic dysfunction (Child-Pugh Class A, B or C). The pharmacokinetics of berotralstat were unchanged in patients with mild hepatic impairment compared to patients with normal hepatic function. In patients with moderate hepatic impairment, Cmax was increased by 77%, while AUC0-inf was increased by 78%. In subjects with severe hepatic impairment, Cmax was increased by 27%, while AUC0-inf was decreased by 6%. The estimated increase in mean QTcF in patients with moderate to severe hepatic dysfunction was up to 8.8 msec (2 sided 90% UB 13.1 msec). Use of berotralstat should be avoided in patients with moderate or severe hepatic impairment (Child-Pugh Class B or C).
Elderly
Berotralstat has not been studied in patients above 75 years of age; however, age is not expected to affect exposure to berotralstat.
5.3. Preclinical safety data
In non-clinical chronic repeat-dose toxicity studies, phospholipidosis (presence of foamy vacuolated macrophages) was observed in the liver of rats (by electron microscopy) and suspected in the liver, small intestine, lung, spleen and lymphoid tissue in rats and monkeys, at clinically relevant exposures. The clinical relevance of these findings is unknown.
Skeletal myofiber degeneration/necrosis was observed in the 2-year (lifetime) study in rats. Exposure at the no observed adverse effect level (NOAEL) for these findings in rats was 4.5 times the exposure achieved (on an AUC basis) at the clinical 150 mg berotralstat dose.
Non-clinical data reveal no special hazard for humans based on conventional studies of genotoxicity.
There was no increase in tumours in a 6-month study in Tg rasH2 transgenic mice. Exposure in this mouse carcinogenicity study was 10 times the exposure achieved (on an AUC basis) at the clinical 150 mg berotralstat dose.
Rare stromal sarcomas of the endometrium and undifferentiated sarcomas of the skin were found in a 2-year (lifetime) study in rats administered berotralstat at an exposure that was 4.5 times the exposure achieved (on an AUC basis) at the clinical 150 mg berotralstat dose. These findings are inconclusive, with an incidence slightly higher than in control groups. The clinical relevance of these findings is unknown.
Berotralstat crossed the placental barrier in rats and rabbits. An embryo-foetal development study conducted in pregnant rats administered berotralstat at exposures 9.7 times the exposure achieved (on an AUC basis) at the clinical 150 mg berotralstat dose revealed no evidence of harm to the developing foetus. A second embryo-foetal development study in a relevant non-rodent species was not conducted.
Berotralstat was detected in the plasma of rat pups on lactation day 14 at approximately 5% of the maternal plasma concentration.
Berotralstat had no effects on mating or fertility in male and female rats at a dose 2.9 times the clinical 150 mg berotralstat dose on a mg/m² basis.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.