PACIMOL Uncoated tablet Ref.[50222] Active ingredients: Paracetamol
Source: Medicines and Medical Devices Safety Authority (NZ) Revision Year: 2021 Publisher: Ipca Pharma (NZ) Pty Limited, PO Box 74509, Auckland 1546 +64 2136 0880 in New Zealand or +61 3 98856172 in Australia
5.1. Pharmacodynamic properties
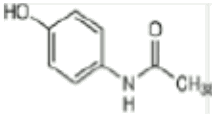
CAS: 103‐90‐2 (paracetamol)
Paracetamol MW 151.17
ATC code Paracetamol, N02BE01
Paracetamol is a para‐aminophenol derivative that exhibits analgesic and anti—pyretic activity. Its mechanism of action is believed to include inhibition of prostaglandin synthesis, primarily within the central nervous system. It is given by mouth or rectally (suppositories) for mild to moderate pain and fever.
The lack of peripheral prostaglandin inhibition confers important pharmacological properties such as the maintenance of the protective prostaglandins within the gastrointestinal tract. Paracetamol is, therefore, particularly suitable for patients with a history of disease or on concomitant medication, where peripheral prostaglandin inhibition would be undesirable (such as, for example, those with a history of gastrointestinal bleeding or the elderly).
5.2. Pharmacokinetic properties
Absorption
Paracetamol is rapidly and almost completely absorbed from the gastrointestinal tract. Food intake delays paracetamol absorption.
Distribution
Paracetamol is distributed into most body tissues. Binding to the plasma proteins is minimal at therapeutic concentrations but increases with increasing doses.
Metabolism
Paracetamol is metabolised in the liver and excreted in the urine mainly as glucuronide and sulphate conjugates.
The metabolites of paracetamol include a minor hydroxylated intermediate which has hepatotoxic activity. This intermediate metabolite is detoxified by conjugation with glutathione. However, it can accumulate following paracetamol overdosage (more than 200 mg/kg or 10 g total paracetamol ingested) and, if left untreated, can cause irreversible liver damage.
Paracetamol is metabolised differently by infants and children compared to adults, the sulphate conjugate being predominant.
Excretion
Paracetamol is excreted in the urine mainly as the glucuronide and sulphate conjugates. Less than 5% is excreted as unmodified paracetamol with 85% to 90% of the administered dose eliminated in the urine within 24 hours of ingestion. The elimination half‐life varies from one to three hours. The mean plasma half‐life is about 2.3 hours.
5.3. Preclinical safety data
N/A.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.