PANADOL BABY Oral suspension Ref.[108763] Active ingredients: Paracetamol
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2023 Publisher: Haleon Ireland Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland
4.1. Therapeutic indications
Panadol Baby suspension is recommended for the relief of pains of teething, toothache and sore throats and for reducing the fever often associated with colds and ‘flu’ and childhood infections such as chicken pox, whooping cough, measles and mumps.
4.2. Posology and method of administration
This product is intended for oral use in children ages:
| Age: 2 – 3 months | Dose |
|---|---|
| 1. Post-vaccination fever | 2.5 mL If necessary, after 4-6 hours, give a second 2.5 mL dose |
| 1. Other causes of pain and fever only if • Weighs over 4 kg • Born after 37 weeks | |
| • Do not give to babies less than 2 months of age • Do not give more than 2 doses • Leave at least 4 hours between doses • If further doses are needed, talk to your doctor or pharmacist |
| Child’s Age | How Much | How often (in 24 hours) |
|---|---|---|
| 3 – 6 months | 2.5 mL | 4 times |
| 6 – 24 months | 5 mL | 4 times |
| 2 – 4 years | 7.5 mL | 4 times |
| 4 – 8 years | 10 mL | 4 times |
| 8 – 10 years | 10 mL + 5 mL | 4 times |
| 10 – 12 years | 10 mL + 10 mL | 4 times |
| • Do not give more than 4 doses in any 24 hour period • Leave at least 4 hours between doses • The lowest dose necessary to achieve efficacy should be used. • Do not give this medicine to your child for more than 3 days without speaking to your doctor orpharmacist |
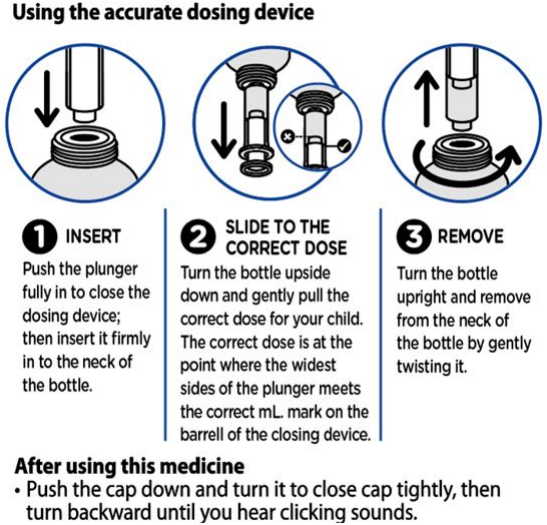
It is important to shake the bottle for at least 10 seconds before use.
If your baby was born prematurely and is less than 3 months old consult your doctor prior to use.
Method of Administration
Panadol Baby suspension is for oral administration only.
After use you should clean the device with warm water and dry it, no need for sterilisation of the device.
4.9. Overdose
Paracetamol overdose may cause liver failure which can lead to liver transplant or death. Acute pancreatitis has been observed, usually with hepatic dysfunction and liver toxicity
There is a risk of poisoning with paracetamol particularly in elderly subjects, young children, patients with liver disease, cases of chronic alcoholism and in patients with chronic malnutrition. Overdosing may be fatal in these cases.
Symptoms generally appear within the first 24 hours and may comprise: nausea, vomiting, anorexia, pallor, and abdominal pain, or patients may be asymptomatic.
Overdose of paracetamol in a single administration in adults or in children can cause liver cell necrosis likely to induce complete and irreversible necrosis, resulting in hepatocellular insufficiency, metabolic acidosis and encephalopathy which may lead to coma and death. Simultaneously, increased levels of hepatic transaminases (AST, ALT), lactate dehydrogenase and bilirubin are observed together with increased prothrombin levels that may appear 12 to 48 hours after administration.
Liver damage is likely in adults who have taken more than the recommended amounts of paracetamol. It is considered that excess quantities of toxic metabolite (usually adequately detoxified by glutathione when normal doses of paracetamol are ingested), become irreversibly bound to liver tissue.
Some patients may be at increased risk of liver damage from paracetamol toxicity.
Risk Factors include: If the patient;
- Is on long-term treatment with carbamazepine, phenobarbitone, phenytoin, primidone, rifampicin, St John’s Wort or other drugs that induce liver enzymes.
- Regularly consumes ethanol in excess of recommended amounts
- Is likely to be glutathione depleted e.g. eating disorders, cystic fibrosis, HIV infection, starvation, cachexia
Emergency Procedure
Immediate transfer to hospital.
Blood sampling to determine initial paracetamol plasma concentration. In the case of a single acute overdose, paracetamol plasma concentration should be measured 4 hours post ingestion.
Administration of activated charcoal should be considered if >150mg/kg paracetamol has been taken within 1 hour.
The antidote N-acetylcysteine, should be administered as soon as possible in accordance with National treatment guidelines.
Symptomatic treatment should be implemented.
6.3. Shelf life
2 years.
6.4. Special precautions for storage
Do not store above 25°C. Do not freeze.
6.5. Nature and contents of container
Amber glass bottles fitted with Clic-loc closures with expanded polyethylene/polypropylene liners. Pack sizes: 60ml, 100ml, 140ml and 200ml.
10 mL plastic dosing syringe consists of a barrel (body) & plunger (slider) made from Polyethylene and Polypropylene plastic grades.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
No special requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.