PANZYGA Solution for injection Ref.[10905] Active ingredients: Human normal immunoglobulin G Immunoglobulins, normal human, IV
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
Immune Globulin Intravenous (Human), PANZYGA, is a solvent/detergent (S/D)-treated, sterile preparation of highly purified immunoglobulin G (IgG) derived from large pools of human plasma. PANZYGA is a solution for infusion to be administered intravenously.
This preparation contains approximately 100 mg of protein per mL (10%), of which not less than 96% is normal human immunoglobulin G. PANZYGA contains not more than 3% aggregates, not less than 90% monomers and dimers, and not more than 3% fragments. On average, the product contains 100 µg/mL of IgA, and lower amounts of IgM.
PANZYGA contains only trace amounts of sodium, and the pH is between 4.5 and 5.0. The osmolality is in the range of 240-310 mosmol/kg.
The manufacturing process for PANZYGA isolates IgG without additional chemical or enzymatic modification, and the Fc portion is maintained intact. PANZYGA contains the IgG antibody activities present in the donor population. IgG subclasses are fully represented with the following approximate percents of total IgG: IgG1 is 65%, IgG2 is 28%, IgG3 is 3% and IgG4 is 4%.
PANZYGA contains a broad spectrum of IgG antibodies against bacterial and viral agents that are capable of opsonization and neutralization of microbes and toxins. PANZYGA contains glycine (15.0-19.5 mg/mL), but no preservatives or sucrose.
All units of human plasma used in the manufacture of PANZYGA are provided by FDA-approved blood and plasma establishments, and are tested by FDA-licensed serological tests for HBsAg, antibodies to HCV and HIV and Nucleic Acid Test (NAT) for HCV and HIV1 and found to be non-reactive (negative).
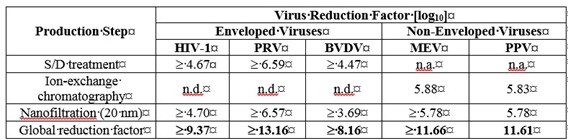
The product is manufactured by the cold ethanol fractionation process followed by purification methodologies, as well as S/D treatment and nanofiltration (20 nm). The S/D mixture used is composed of tri-n-butyl phosphate (TNBP, solvent) and Triton X-100 (Octoxynol, detergent). The PANZYGA manufacturing process shows significant viral reduction and inactivation, demonstrated by in vitro infectivity studies (Table 4). The virus safety of PANZYGA is achieved through a combination of various process steps, including S/D treatment, ion-exchange chromatography, and nanofiltration (20 nm).
Table 4 shows the virus clearance during the manufacturing process for PANZYGA, expressed as the mean log10 reduction factor (LRF).
Table 4. Virus Reduction by PANZYGA Manufacturing Process:
HIV-1: Human Immunodeficiency Virus – 1, a model for HIV-1 and HIV-2;
PRV: Pseudorabies Virus, a model for large enveloped DNA viruses (e.g., herpes virus);
BVDV: Bovine Viral Diarrhea Virus, a model for e.g., Hepatitis C virus (HCV) and West-Nile virus (WNV);
MEV: Mouse Encephalomyelitis virus, a model for Hepatitis A virus (HAV);
PPV: Porcine Parvovirus, a model for Human Parvovirus B19;
n.a.: not applicable;
n.d: not done.
Additionally, the manufacturing process was investigated for its capacity to decrease the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the vCJD and CJD agents.[10]
Several of the individual production steps in the PANZYGA manufacturing process were shown to decrease TSE infectivity of that experimental model agent. TSE reduction steps include ion-exchange chromatography and nanofiltration, which together give a total of at least 10.4 log10 decrease of infectivity. These studies provide reasonable assurance that low levels of CJD/vCJD agent infectivity, if present in the starting material, would be removed.
| Dosage Forms and Strengths |
|---|
|
Solution containing 10% IgG (100 mg/mL) (See How Supplied/Storage and Handling (16)). |
| How Supplied | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
PANZYGA is supplied in 1 g, 2.5 g, 5 g, 10 g, 20 g, and 30 g single-use bottles. The table below shows the details of available presentations of PANZYGA.
PANZYGA is not supplied with an infusion set. If a filtered infusion set is used (not mandatory), choose a filter size of 0.2-200 microns. Components used in the packaging of PANZYGA are not made with natural rubber latex. Manufactured by: Octapharma Pharmazeutika Produktionsges.m.b.H., Oberlaaer Strasse 235, 1100 Vienna, Austria Distributed by: Pfizer Labs, Division of Pfizer Inc, NY, NY 10017 |
Drugs
| Drug | Countries | |
|---|---|---|
| PANZYGA | Austria, Canada, Ecuador, Estonia, Spain, Ireland, Lithuania, Malta, Netherlands, Poland, Romania, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.