PANZYGA Solution for injection Ref.[10905] Active ingredients: Human normal immunoglobulin G Immunoglobulins, normal human, IV
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
Treatment of Primary Humoral Immunodeficiency (PI)
PANZYGA supplies a broad spectrum of opsonic and neutralizing IgG antibodies against bacteria or their toxins. The mechanism of action in PI has not been fully elucidated.
Treatment of Chronic Immune Thrombocytopenia (ITP) in Adults
The mechanism of action of immunoglobulins in the treatment of chronic ITP in adults has not been fully elucidated.
Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) in Adults
The mechanism of action of immunoglobulins in the treatment of CIDP in adults has not been fully elucidated.
12.2. Pharmacodynamics
PANZYGA contains mainly immunoglobulin G (IgG) with a broad spectrum of antibodies against various infectious agents reflecting the IgG activity found in the donor population. PANZYGA which is prepared from pooled material from not less than 1000 donors, has an IgG subclass distribution similar to that of native human plasma. Adequate doses of IGIV can restore abnormally low IgG level to the normal range. Standard pharmacodynamic studies were not performed.
12.3. Pharmacokinetics
Treatment of Primary Humoral Immunodeficiency (PI)
In the PI study, 50 pediatric and adult subjects underwent pharmacokinetic assessments. Subjects received infusions of PANZYGA (200 to 800 mg/kg body weight) every 3 or 4 weeks for 12 months. Blood samples for PK study were collected between the 7th and 9th PANZYGA infusion, depending on the individual treatment schedule.
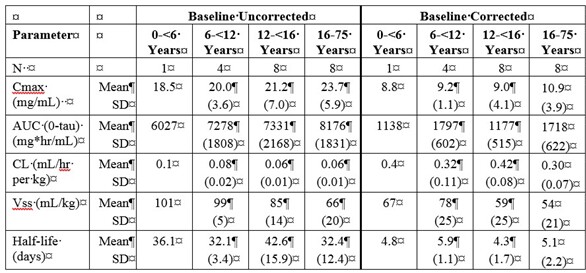
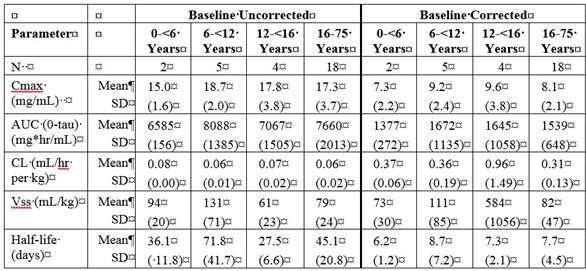
Table 5a and 5b summarize the pharmacokinetic parameters of PANZYGA, based on serum concentrations of total IgG, in subjects receiving infusions every 3, or 4 weeks, respectively.
Table 5. PI Study - Pharmacokinetic Parameters of PANZYGA in Subjects:
a) PK Parameters: IgG Arm: 3-weeks
b) PK Parameters: IgG Arm: 4-weeks
Treatment of Chronic Immune Thrombocytopenia (ITP) in Adults
Pharmacokinetic studies with PANZYGA have not been performed in adults with chronic ITP.
Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) in Adults
Pharmacokinetic studies with PANZYGA have not been performed in adult patients with CIDP.
The IgG trough levels were evaluated at each visit (Table 6) before the PANZYGA infusion. There was the option of rescue treatment with two consecutive infusions of 2.0 g/kg Panzyga at 3-week intervals (±4 days) for all subjects in the 0.5 and 1.0 g/kg Panzyga arms who were either stable at Week 6 or deteriorated after Week 3 and before Week 18. The actual administered dose for the three dose arms was: 0.91 ± 0.4 (n=35), 1.24 ± 0.2 (n=69) and 1.97 ± 0.2 (n=38) g/kg for 0.5, 1 and 2g/kg, respectively. There were no major differences among the three dose arms in demographic characteristics and baseline IgG levels. The percentage of increase in mean IgG from baseline to the end of study assessment was 46% in the 0.5 g/kg arm, 57% in the 1.0 g/kg arm and 91% in the 2.0 g/kg arm (Table 6).
Table 6. Mean IgG trough levels in subjects with CIDP:
| IgG troughs (g/L) | PANZYGA 0.5 g/kg | PANYZGA 1.0 g/kg | PANZYGA 2.0 g/kg | |
|---|---|---|---|---|
| Visit / Time point | (N=35) | (N=69) | (N=38) | |
| Visit 2 - Week 0 | Mean (SD) | 10.6 (3.1) | 10.5 (2.5) | 10.2 (3.0) |
| Visit 3 - Week 3 | Mean (SD) | 17.9 (3.5) | 17.1 (3.1) | 16.5 (3.3) |
| Visit 4 - Week 6 | Mean (SD) | 15.5 (3.0) | 16.5 (3.3) | 18.5 (4.0) |
| Visit 5 - Week 9 | Mean (SD) | 15.6 (3.3) | 16.5 (3.0) | 19.2 (4.4) |
| Visit 6 - Week 12 | Mean (SD) | 14.2 (2.6) | 16.8 (3.8) | 19.6 (4.3) |
| Visit 7 - Week 15 | Mean (SD) | 14.1 (2.7) | 16.2 (3.4) | 19.7 (4.4) |
| Visit 8 - Week 18 | Mean (SD) | 14.1 (2.3) | 15.9 (3.1) | 19.6 (5.2) |
| Visit 9 - Week 21 | Mean (SD) | 14.3 (2.3) | 16.0 (3.0) | 18.9 (3.5) |
| End of Study - Week 24 | Mean (SD) | 15.5 (3.6) | 16.5 (3.3) | 19.5 (4.6) |
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies were conducted on carcinogenesis, mutagenesis, or impairment of fertility with PANZYGA.
13.2. Animal Toxicology and/or Pharmacology
Several standard nonclinical proof-of-concept and safety studies were performed with PANZYGA in animals. These included acute toxicity, pharmacokinetic, local tolerance, and safety pharmacology studies. PANZYGA. There were no adverse effects attributed to PANZYGA in the animal studies.
TNBP and Octoxynol-9 may be found in PANZYGA in trace amounts. In single- and repeated-dose toxicity studies in animals, these impurities caused no adverse effects when administered (alone or in combination) at doses multiple times higher than the equivalent human dose. A mixture of these compounds did not show teratogenic effects when administered to pregnant rabbits and rats during organogenesis.
14. Clinical Studies
14.1 Treatment of Primary Humoral Immunodeficiency (PI)
Study 1: In a prospective, open-label, single-arm, multicenter study in 51 children and adults with PI, subjects received PANZYGA at a dose between 200 to 800 mg/kg body weight every 3 or 4 weeks. Subjects participated in the study for a mean of 360 days. Infusions were initiated at a rate of 1 mg/kg/min for the first 30 minutes, and, if tolerated, could be advanced to a maximum tolerated rate not exceeding 8 mg/kg/min. The mean age of subjects was 26.8 years (range: 2 to 65 years).
The primary efficacy endpoint was the number of episodes of serious bacterial infections per patient per year. Serious infection included pneumonia, bacteremia or sepsis, osteomyelitis/septic arthritis, visceral abscesses, or bacterial meningitis. Secondary efficacy variables included: occurrence of any infection of any kind or seriousness; time to resolution of infections; use of antibiotics; the number of days of work/school missed; the number and days of hospitalizations; and the number of episodes of fever.
For the primary endpoint, the observed rate was 0.08 serious bacterial infections per patient per year (4 infections over 50.2 patient-years).
Only 1 adult patient was hospitalized due to an infection for 4 days (overall rate of days in hospital per person-year: 0.080). Episodes of fever were observed for less than 25% of all patients. The mean resolution time was 14 days for serious bacterial infections and 18 days for other infections. Approximately 50% of all patients missed at least 1 day of work or school due to infections, with an annual rate of less than 4 days/person-year.
Table 7 summarizes the efficacy results for all 51 subjects.
Table 7. Study 1 – Summary of Efficacy Results for subjects with PI:
| Category | Result | Unit |
|---|---|---|
| Number of subjects | 51 | Subjects |
| Total number of subject days | 18,349 | Days |
| Annual rate of confirmed serious bacterial infections (SBIs)* | 0.080 | SBIs/person-year** |
| Annual rate of other infections | 3.682 | Inf./person-year |
| Number of subjects (%) with use of antibiotics | 42 (82.4%) | Subjects(%) |
| Annual rate of use of antibiotics | 87 | Days/person-year |
| Absences from work or school due to Infection, number of days (%) | 183 (1.0%) | Days (%) |
| Annual rate of absences from work or school due to infection | 3.6 | Days/person-year |
| Hospitalization due to infection, number of days | 4 | Days |
| Annual rate of hospitalizations due to infection | 0.1 | Day/person-year |
* Defined as bacteremia/sepsis, bacterial meningitis, osteomyelitis/septic arthritis, bacterial pneumonia and visceral abscess
** Upper 1-sided 99% confidence interval: 0.503
Throughout the entire study, the serum IgG trough levels were nearly constant for both treatment schedules and were above the required trough levels of about 5-6 g/L. The calculated pharmacokinetic parameters showed that the minimum concentration of IgG was at least 6.8 g/L for both treatment intervals.
14.2 Treatment of Chronic Immune Thrombocytopenia (ITP) in Adults
A prospective, open-label, single-arm, multicenter study assessed the efficacy, safety, and tolerability of PANZYGA in 40 subjects with chronic ITP and a platelet count of 20 x 109/L or less. Subjects ranged in age from 18 to 72 years (median: 32 years); 43% were female and 57% were male. Ninety percent of the subjects were Caucasian and 10% were Asian.
Subjects received a 2 g/kg dose of PANZYGA administered as two daily 1 g/kg intravenous doses, given on 2 consecutive days. All but one patient received the maximum infusion rate of 8 mg/kg/minute, starting at 1 mg/kg/minute. Platelet counts were measured on Days 1 to 8, 15, and 22.
The study was designed to determine the response rate, defined as the percentage of subjects with an increase in platelet count to at least 50 x 109/L within 7 days after the first infusion (responders). Additionally, maximum platelet count, the time to reach a platelet count of at least 50 x 109/L within the first 7 days, the duration of that response (i.e., the number of days the platelet count remained in excess of 50 x 109/L), and the regression of hemorrhages in subjects who had bleeding at baseline were observed.
Of the 36 subjects in the full analysis set, 29 (81%: 95% CI: 64%- 92%).) responded to PANZYGA with a rise in platelet count to at least 50 x 109/L within 7 days after the first infusion. The lower bound of the overall 95% confidence interval for the response rate in all 36 subjects (64%) is above the predefined response rate of 60%.
Table 8 shows the median and mean of the maximum platelet count.
Table 8. Maximum Platelet Count (x109/L):
| ITP subjects (n=36) | |
|---|---|
| Median and range | 196 (8 to 1067) |
| Mean ± standard deviation | 237 ± 205 |
Table 9 shows the median and mean of the time to and duration of platelet response.
Table 9. Time to and Duration of Platelet Response (Responders Only):
| Time to Platelet Response (at least 50x109/L) (Days) | Duration of Platelet Response (Days) | |
|---|---|---|
| ITP Subjects Responders (n=29) | ITP Subjects Responders (n=29) | |
| Median and range | 2 (1 to 4) | 14 (1 to 20) |
| Mean ± standard deviation | 1.8 ± 0.8 | 12.4 ± 5.8 |
Of the 36 subjects, 23 (64%) subjects had bleeding at baseline. Bleeding was minor in 14 subjects (39%), mild in 2 subjects (6%) and moderate in 7 subjects (19%). On Day 7, only 14% of subjects were bleeding (5/36). Persistent bleeding was mild in 1 and minor in 2 subjects. Information regarding bleeding resolution was missing in 2 subjects with moderate bleeding.
14.3 Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) in Adults
The efficacy of PANZYGA in adults with CIDP was evaluated in a prospective, double-blind, randomized, multicenter study that enrolled 142 adult subjects (between 18 and 83 years of age) with CIDP who deteriorated in the Wash-out Phase, during which the current medication (immunoglobulins or corticosteroids) was reduced gradually. Subjects were randomized 1:2:1 to receive first a loading dose of 2 g/kg, and then 0.5 g/kg, 1.0 g/kg or 2.0 g/kg PANZYGA according to their respective dose arm for 7 maintenance infusions at 3-week intervals during the 24-week Dose-evaluation Phase. Subjects in the 0.5 g/kg and 1.0 g/kg arms had the option of rescue treatment with two consecutive infusions of 2.0 g/kg Panzyga at 3-week intervals if criteria were met [see CLINICAL PHARMACOLOGY (12.3)].
Efficacy was based on the proportion of responders in the 1.0 g/kg PANZYGA arm at Week 24 relative to Baseline (Week 0). A responder was defined as a subject with a decrease of at least 1 point in the adjusted 10-point Inflammatory Neuropathy Cause and Treatment (INCAT) disability score at Week 24 relative to Baseline. The proportion of responders in the 1.0 g/kg arm was 79.71% (95% CI: 68.8, 87.5), with 55 out of 69 subjects classified as responders. Efficacy was supported by the proportion of responders in the 2.0 g/kg dose arm in the adjusted INCAT disability score, and the proportion of responders in the 1.0 g/kg and 2.0 g/kg dose arms in the grip strength, inflammatory Rasch-built Overall Disability Scale (I-RODS) and Medical Research Council (MRC) sum scores (Table 10).
Table 10. Responder rates for different efficacy scores and dose arms:
| 1.0 g/kg N=69 | 2.0 g/kg N=36 | |
|---|---|---|
| Adjusted INCAT Disability Score | ||
| Number (%) of responders | 55 (79.7%) | 33 (91.7%) |
| 95% CI | 68.8; 87.5 | 78.2; 97.1 |
| I-RODS | ||
| Number (%) of responders | 38 (55.1%) | 26 (72.2%) |
| 95% CI | 43.4; 66.2 | 56; 84.2 |
| Grip Strength | ||
| Number (%) of responders | 45 (65.2%) | 30 (83.3%) |
| 95% CI | 53.4; 75.4 | 68.1; 92.1 |
| MRC Sum Score | ||
| Number (%) of responders | 50 (72.5%) | 31 (86.1%) |
| 95% CI | 61; 81.6 | 71.3; 93.9 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.