PARAPANE OSTEO Film-coated tablet Ref.[50302] Active ingredients: Paracetamol
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2021 Publisher: Beximco Pharmaceuticals Australia, 4 Miami Key, Broadbeach Waters, QLD 4218
Product name and form
PARAPANE OSTEO Paracetamol.
| Pharmaceutical Form |
|---|
|
Parapane Osteo is a bi-layer white colored capsule shaped film coated tablet, engraved BPL on one side and plain on the other. |
Qualitative and quantitative composition
Active ingredient: Paracetamol 665 mg/tablet.
Excipients: For the full list of excipients, see section 6.1 List of Excipients.
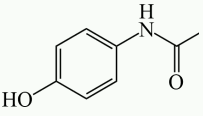
Chemical structure
Chemical name: N-acetyl-paminophenol
Molecular Formula: C8H9NO2
Molecular weight: 151.17
CAS No.: 103-90-2
| Active Ingredient |
|---|
|
Paracetamol is a medication used to treat pain and fever. It does appear to selectively inhibit COX activities in the brain, which may contribute to its ability to treat fever and pain. |
| List of Excipients |
|---|
|
The tablets contain hydroxy ethyl cellulose, microcrystalline cellulose, povidone, pregelatinized starch, magnesium stearate, maize starch, sodium starch glycolate, stearic acid, OPADRY KB low viscosity film coating system 310A180023 WHITE and carnauba wax. The tablets are sugar, lactose and gluten free. |
Pack sizes and marketing
Alu-PVDC blister packs of 24, 48 and 96 tablets.
Marketing authorization holder
Beximco Pharmaceuticals Australia, 4 Miami Key, Broadbeach Waters, QLD 4218
Marketing authorization dates and numbers
01/11/2016
Drugs
| Drug | Countries | |
|---|---|---|
| PARAPANE | Australia, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.