PAXLOVID Film-coated tablet Ref.[49622] Active ingredients: Nirmatrelvir Nirmatrelvir and Ritonavir Ritonavir
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Pfizer Europe MA EEIG, Boulevard de la Plaine 17, 1050 Brussels, Belgium
4.3. Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
Medicinal products that are highly dependent on CYP3A for clearance and for which elevated concentrations are associated with serious and/or life-threatening reactions.
Medicinal products that are potent CYP3A inducers where significantly reduced PF-07321332/ritonavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance.
Paxlovid cannot be started immediately after discontinuation of any of the following medicinal products due to the delayed offset of the recently discontinued CYP3A inducer (see section 4.5).
Medicinal products listed below are a guide and not considered a comprehensive list of all possible medicinal products that are contraindicated with Paxlovid.
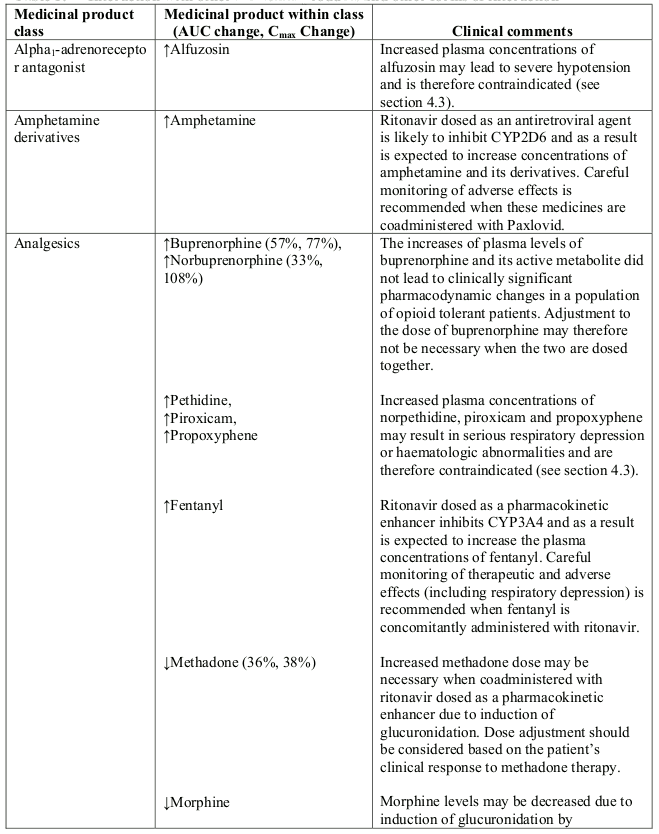
- Alpha1-adrenoreceptor antagonist: alfuzosin
- Analgesics: pethidine, piroxicam, propoxyphene
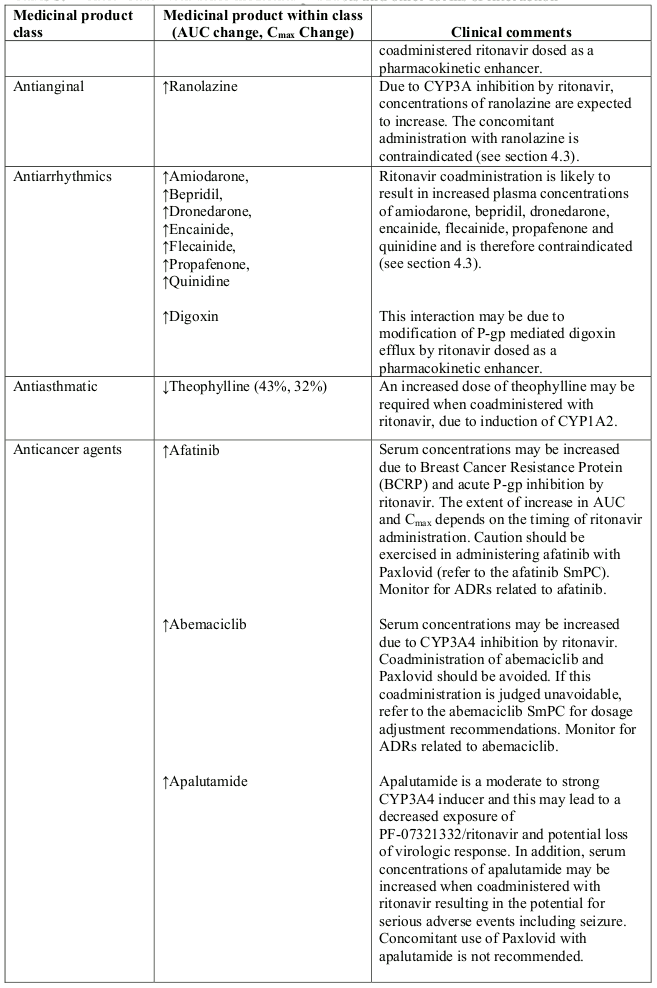
- Antianginal: ranolazine
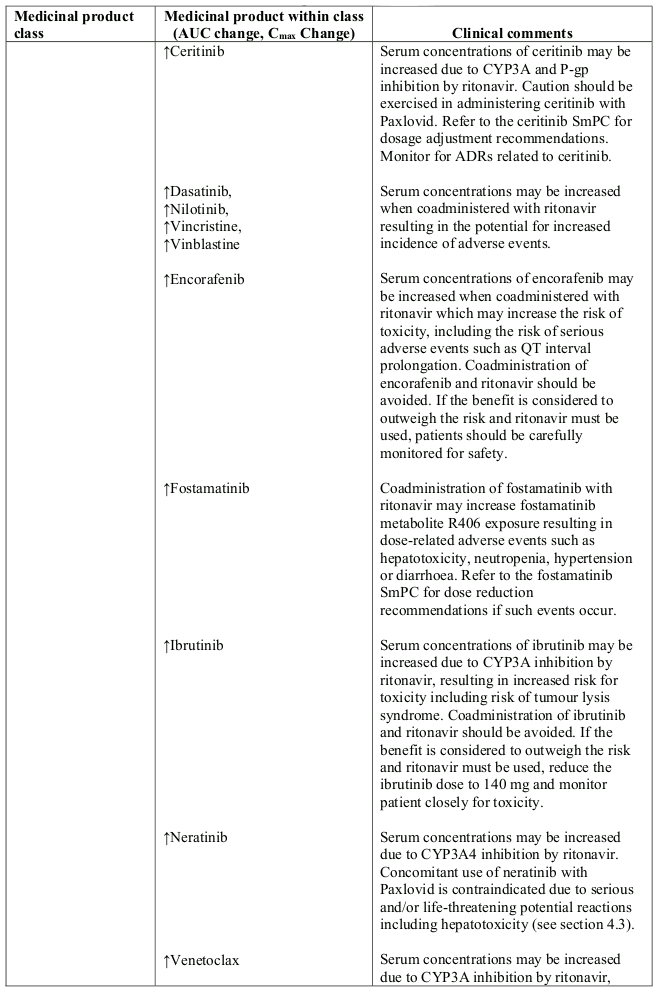
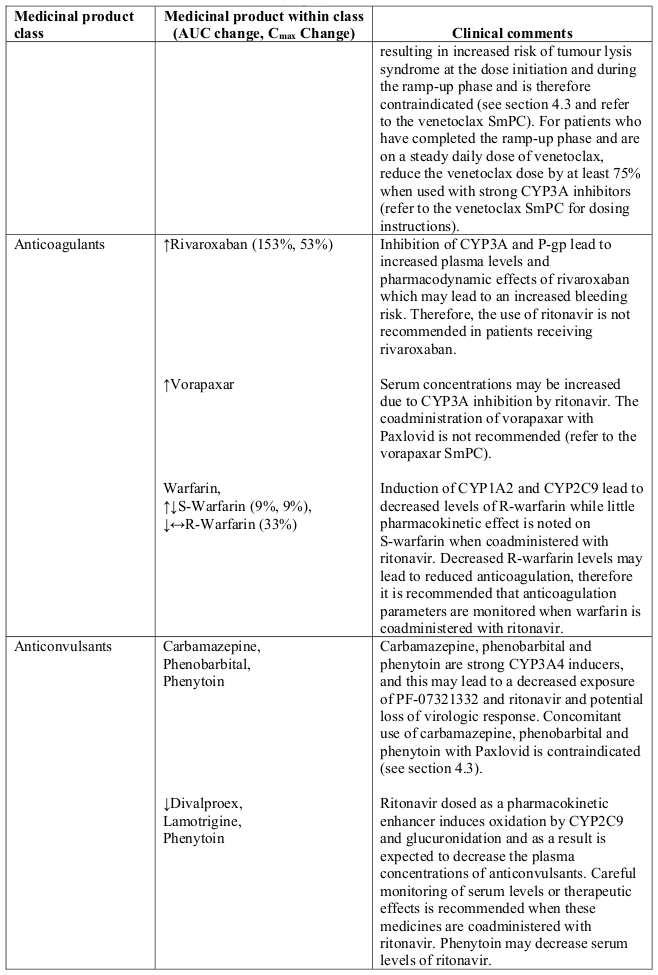
- Anticancer drugs: neratinib, venetoclax
- Antiarrhythmic: amiodarone, bepridil, dronedarone, encainide, flecainide, propafenone, quinidine
- Antibiotics: fusidic acid, rifampicin
- Anticonvulsants: carbamazepine, phenobarbital, phenytoin
- Anti-gout: colchicine
- Antihistamines: astemizole, terfenadine
- Antipsychotics/neuroleptics: lurasidone, pimozide, clozapine, quetiapine
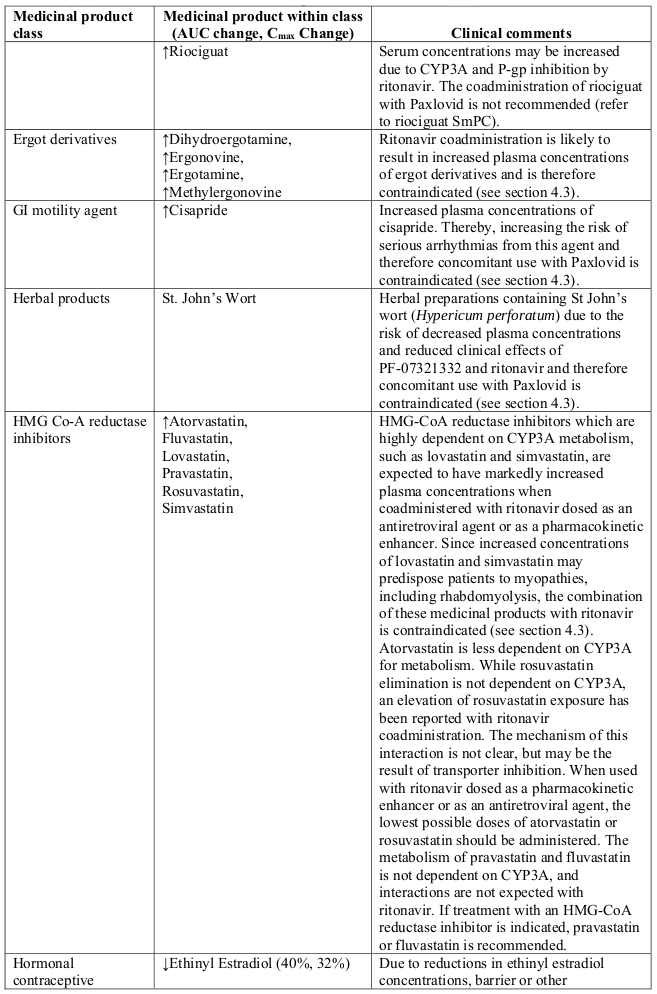
- Ergot derivatives: dihydroergotamine, ergonovine, ergotamine, methylergonovine
- GI motility agents: cisapride
- Herbal products: St. John's wort (Hypericum perforatum)
- Lipid-modifying agents:
- HMG Co-A reductase inhibitors: lovastatin, simvastatin
- Microsomal triglyceride transfer protein (MTTP) inhibitor: lomitapide
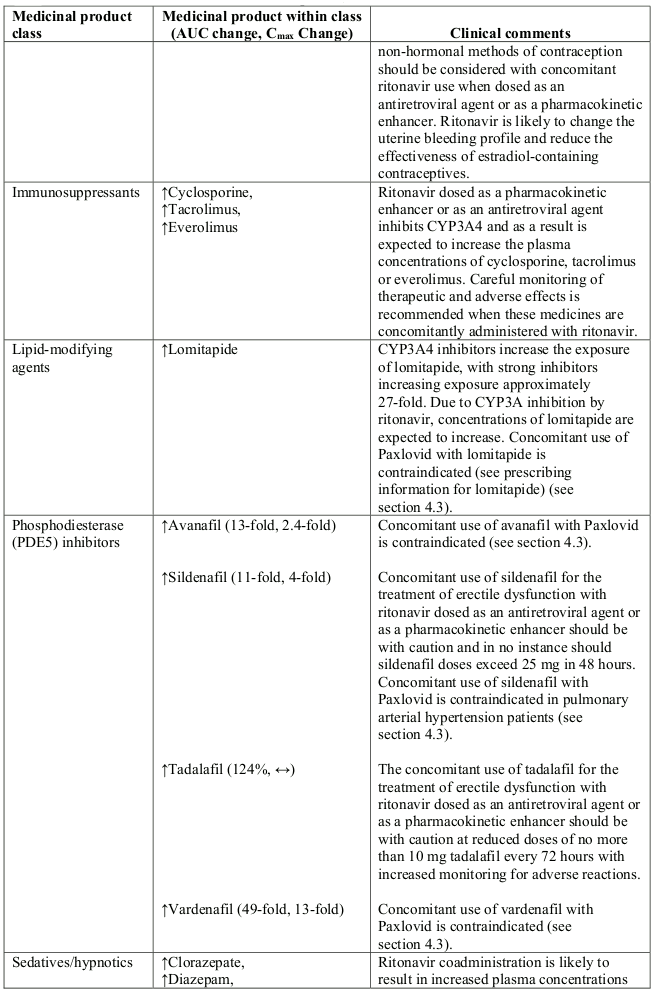
- PDE5 inhibitor: avanafil, sildenafil, vardenafil
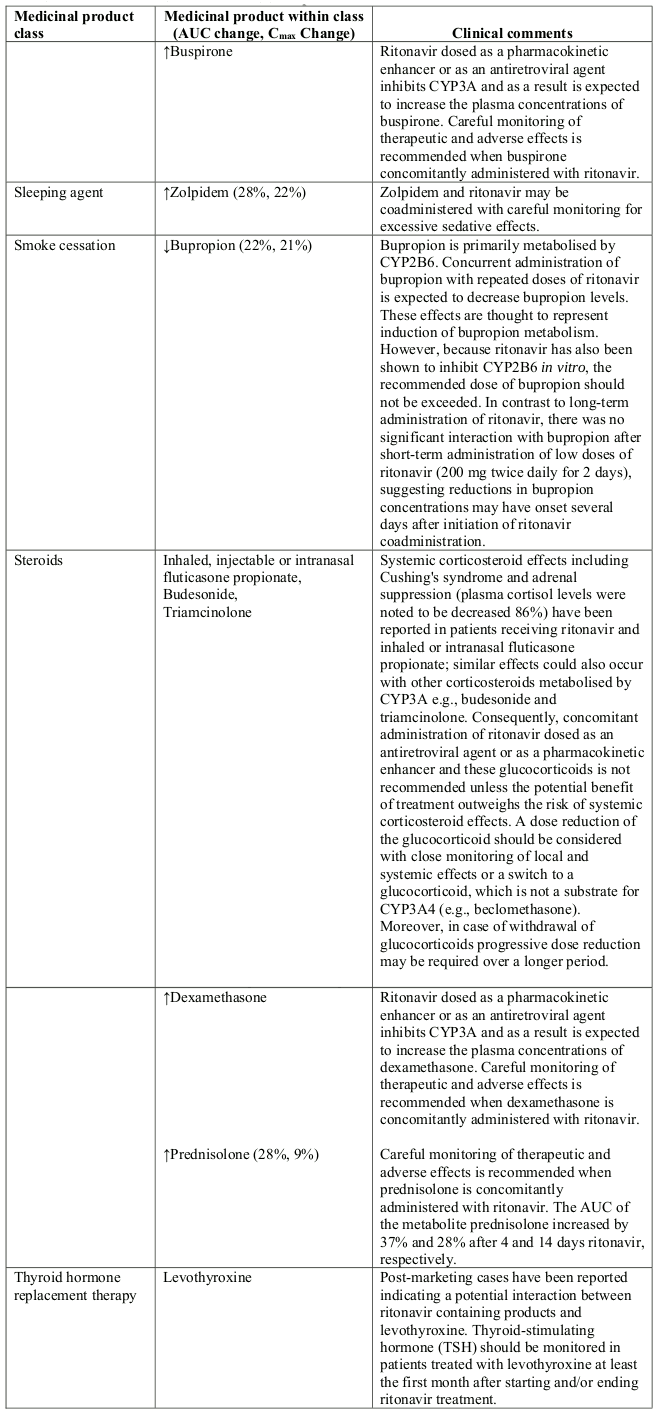
- Sedative/hypnotics: clorazepate, diazepam, estazolam, flurazepam, oral midazolam and triazolam
4.4. Special warnings and precautions for use
Risk of serious adverse reactions due to interactions with other medicinal products
Initiation of Paxlovid, a CYP3A inhibitor, in patients receiving medicinal products metabolised by CYP3A or initiation of medicinal products metabolised by CYP3A in patients already receiving Paxlovid, may increase plasma concentrations of medicinal products metabolised by CYP3A.
Initiation of medicinal products that inhibit or induce CYP3A may increase or decrease concentrations of Paxlovid, respectively.
These interactions may lead to:
- Clinically significant adverse reactions, potentially leading to severe, life-threatening or fatal events from greater exposures of concomitant medicinal products.
- Clinically significant adverse reactions from greater exposures of Paxlovid.
- Loss of therapeutic effect of Paxlovid and possible development of viral resistance.
See Table 1 for medicinal products that are contraindicated for concomitant use with PF-07321332/ritonavir and for potentially significant interactions with other medicinal products (see section 4.5). Potential for interactions should be considered with other medicinal products prior to and during Paxlovid therapy; concomitant medicinal products should be reviewed during Paxlovid therapy and the patient should be monitored for the adverse reactions associated with the concomitant medicinal products.
Severe renal impairment
No clinical data are available in patients with severe renal impairment (including patients with ESRD). Based on pharmacokinetic data (see section 5.2), the use of Paxlovid in patients with severe renal impairment could lead to over-exposure with potential toxicity. No recommendation in terms of dose adjustment could be elaborated at this stage pending dedicated investigation. Therefore, Paxlovid should not be used in patients with severe renal impairment (eGFR <30 mL/min, including patients with ESRD under haemodialysis).
Severe hepatic impairment
No pharmacokinetic and clinical data are available in patients with severe hepatic impairment. Therefore, Paxlovid should not be used in patients with severe hepatic impairment.
Hepatotoxicity
Hepatic transaminase elevations, clinical hepatitis and jaundice have occurred in patients receiving ritonavir. Therefore, caution should be exercised when administering Paxlovid to patients with pre-existing liver diseases, liver enzyme abnormalities or hepatitis.
Risk of HIV-1 resistance development
Because PF-07321332 is coadministered with ritonavir, there may be a risk of HIV-1 developing resistance to HIV protease inhibitors in individuals with uncontrolled or undiagnosed HIV-1 infection.
Excipients
PF-07321332 tablets contain lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine.
PF-07321332 and ritonavir tablets each contain less than 1 mmol sodium (23 mg) per dose, that is to say essentially 'sodium-free'.
4.5. Interaction with other medicinal products and other forms of interaction
Paxlovid (PF-07321332/ritonavir) is an inhibitor of CYP3A and may increase plasma concentrations of medicinal products that are primarily metabolised by CYP3A. Medicinal products that are extensively metabolised by CYP3A and have high first pass metabolism appear to be the most susceptible to large increases in exposure when coadministered with PF-07321332/ritonavir. Thus, coadministration of PF-07321332/ritonavir with medicinal products highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events is contraindicated (see Table 1).
Ritonavir has a high affinity for several cytochrome P450 (CYP) isoforms and may inhibit oxidation with the following ranked order: CYP3A4 > CYP2D6. Ritonavir also has a high affinity for P-glycoprotein (P-gp) and may inhibit this transporter. Ritonavir may induce glucuronidation and oxidation by CYP1A2, CYP2C8, CYP2C9 and CYP2C19 thereby increasing the biotransformation of some medicinal products metabolised by these pathways and may result in decreased systemic exposure to such medicinal products, which could decrease or shorten their therapeutic effect.
Coadministration of other CYP3A4 substrates that may lead to potentially significant interaction (see Table 1) should be considered only if the benefits outweigh the risks.
PF-07321332 and ritonavir are CYP3A substrates; therefore, medicinal products that induce CYP3A may decrease PF-07321332 and ritonavir plasma concentrations and reduce Paxlovid therapeutic effect.
As a conservative measure, the drug-drug interactions pertaining to ritonavir used in chronic HIV infection (600 mg BID when originally used as an antiretroviral agent and 100 mg BID as currently used as a pharmacokinetic enhancer with antiretroviral agents), should apply for Paxlovid. Future investigations may enable to adjust the recommendations related to drug-drug interactions to the 5 days treatment duration of Paxlovid.
Medicinal products listed in Table 1 are a guide and not considered a comprehensive list of all possible medicinal products that are contraindicated or may interact with PF-07321332/ritonavir.
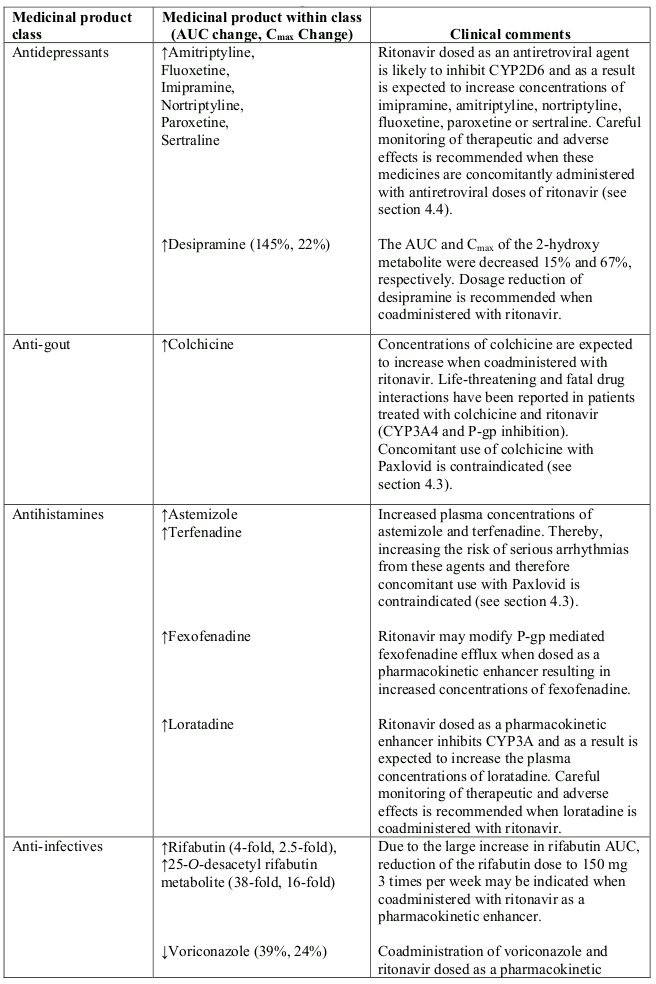
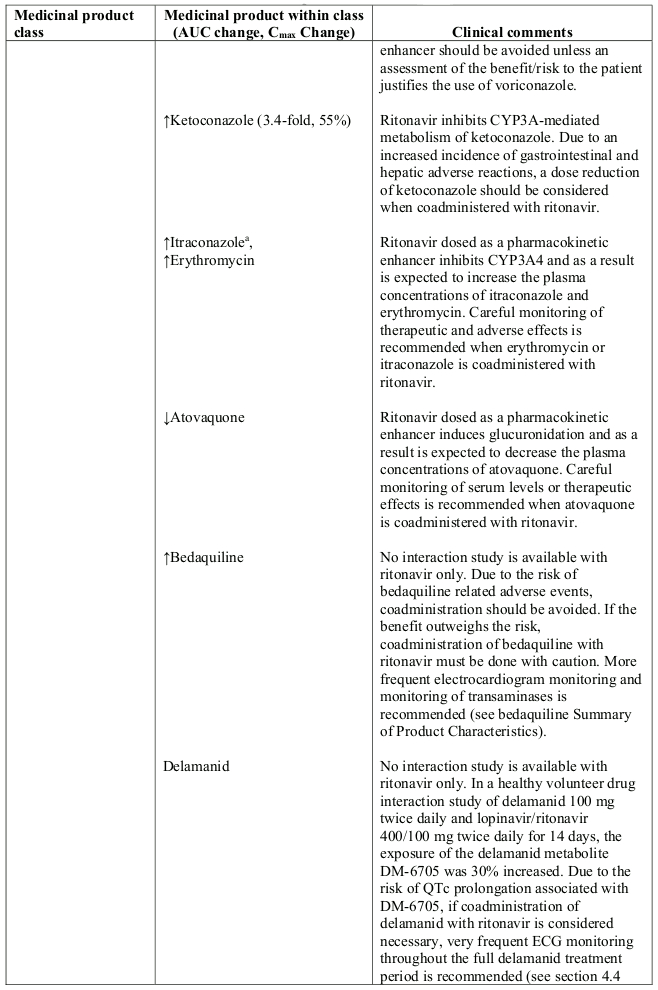
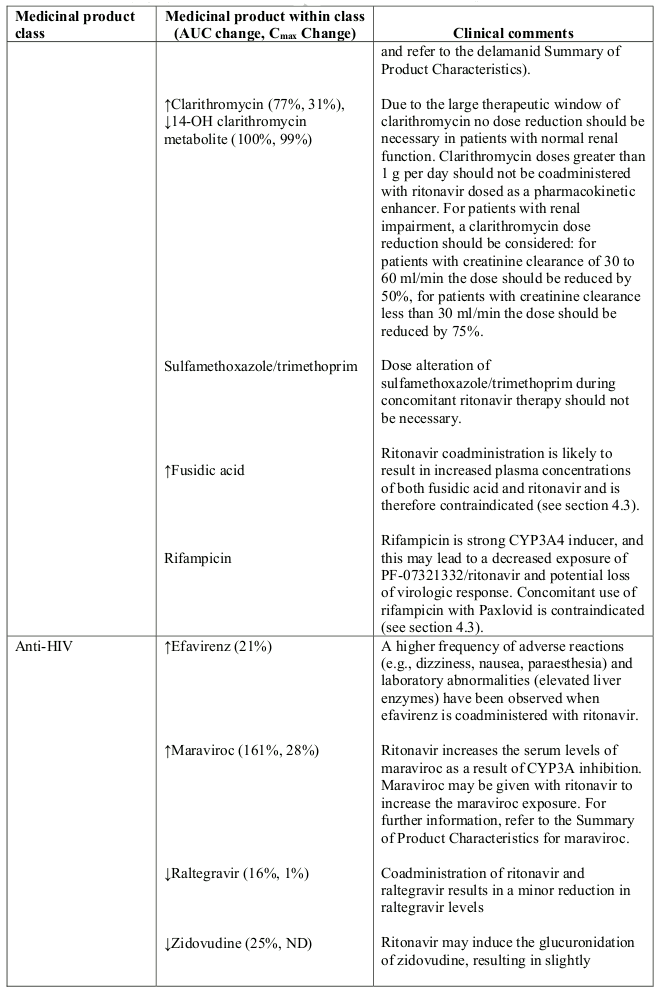
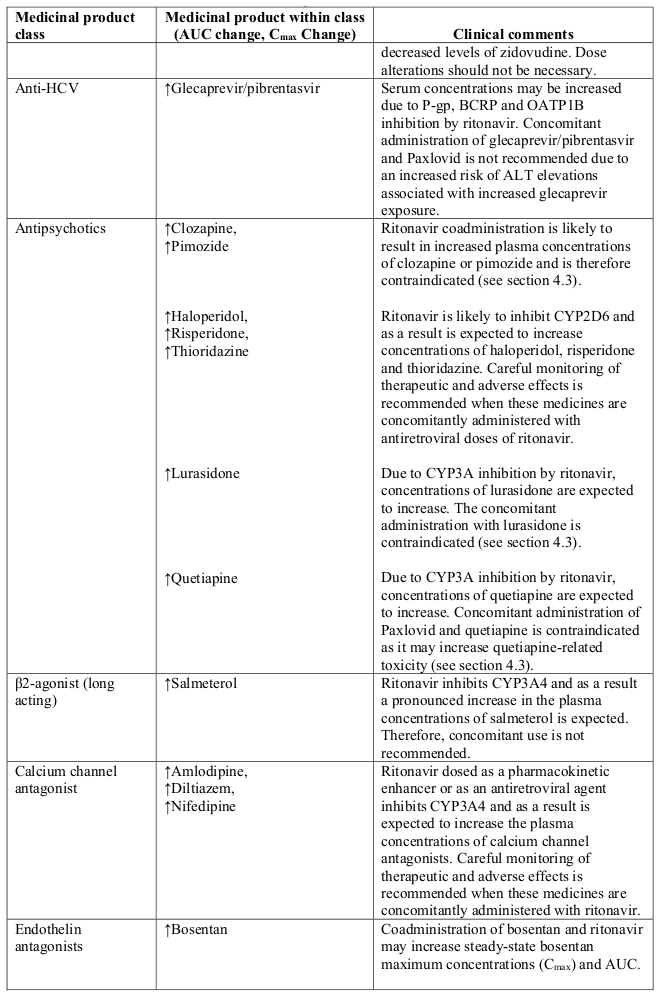
Table 1. Interaction with other medicinal products and other forms of interaction:
Abbreviations: ATL=alanine aminotransferase; AUC=area under the curve.
Effect of other medicinal products on PF-07321332
Coadministration of multiple oral 200 mg doses of itraconazole increased PF-07321332 AUCtau and Cmax. The ratios of the adjusted geometric means (90% CI) for PF-07321332 AUCtau and Cmax were 138.82% (129.25%, 149.11%) and 118.57% (112.50%, 124.97%), respectively, when PF-07321332/ritonavir was coadministered with multiple doses of itraconazole as compared to PF-07321332/ritonavir administered alone.
Coadministration of multiple oral 300 mg doses of carbamazepine decreased PF-07321332 AUCinf and Cmax. The ratios of the adjusted geometric means for PF-07321332 AUCinf and Cmax (90% CI) were 44.50% (90% CI: 33.77%, 58.65%) and 56.82% (90% CI: 47.04%, 68.62%), respectively, following PF-07321332/ritonavir 300 mg/100 mg coadministration with multiple oral doses of carbamazepine compared to PF-07321332/ritonavir administered alone.
4.6. Fertility, pregnancy and lactation
Women of childbearing potential
There are no data on the use of Paxlovid in pregnant women to inform the drug-associated risk of adverse developmental outcomes; women of childbearing potential should avoid becoming pregnant during treatment with Paxlovid and as a precautionary measure for 7 days after completing Paxlovid.
Use of ritonavir may reduce the efficacy of combined hormonal contraceptives. Patients using combined hormonal contraceptives should be advised to use an effective alternative contraceptive method or an additional barrier method of contraception during treatment with Paxlovid, and until one menstrual cycle after stopping Paxlovid (see section 4.5).
Pregnancy
There are no data from the use of Paxlovid in pregnant women.
There was no PF-07321332-related effect on foetal morphology or embryo-foetal viability at any dose tested in rat or rabbit embryo-foetal developmental toxicity studies although lower foetal body weights were observed in rabbit (see section 5.3).
A large number of women exposed to ritonavir during pregnancy indicate no increase in the rate of birth defects compared to rates observed in population-based birth defect surveillance systems.
Animal data with ritonavir have shown reproductive toxicity (see section 5.3).
Paxlovid is not recommended during pregnancy and in women of childbearing potential not using contraception unless the clinical condition requires treatment with Paxlovid.
Breast-feeding
There are no data on the use of Paxlovid in breast-feeding women.
It is unknown whether PF-07321332 is present in human or animal milk, and the effects of it on the breast-fed newborn/infant, or the effects on milk production. Limited published data reports that ritonavir is present in human milk. There is no information on the effects of ritonavir on the breast-fed newborn/infant or on milk production. A risk to the newborn/infant cannot be excluded. Breast-feeding should be discontinued during treatment and as a precautionary measure for 7 days after completing Paxlovid.
Fertility
There are no human data on the effect of Paxlovid (PF-07321332 and ritonavir) or ritonavir alone on fertility. Both PF-07321332 and ritonavir, tested separately, produced no effects on fertility in rats (see section 5.3).
4.7. Effects on ability to drive and use machines
Paxlovid is expected to have no influence on the ability to drive and use machines.
4.8. Undesirable effects
Summary of the safety profile
The most common adverse reactions reported during treatment with Paxlovid (PF-07321332/ritonavir 300 mg/100 mg) every 12 hours for 5 days and during 34 days after the last dose were dysgeusia (5.6%), diarrhoea (3.1%), headache (1.4%) and vomiting (1.1%).
Tabulated summary of adverse reactions
The adverse reactions in Table 2 are listed below by system organ class and frequency. Frequencies are defined as follows: Very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); not known (frequency cannot be estimated from the available data).
Table 2. Adverse reactions with Paxlovid:
| System organ class | Frequency category | Adverse reactions |
|---|---|---|
| Nervous system disorders | Common | Dysgeusia, headache |
| Gastrointestinal disorders | Common | Diarrhoea, vomiting |
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.