PRANDIN Tablet Ref.[27459] Active ingredients: Repaglinide
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
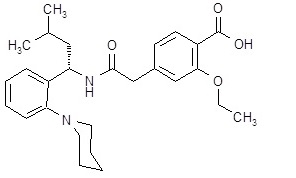
PRANDIN (repaglinide) is an oral blood glucose-lowering drug of the glinide class. Repaglinide, S(+)2-ethoxy-4(2((3-methyl-1-(2-(1 piperidinyl) phenyl)-butyl) amino)-2-oxoethyl) benzoic acid, is chemically unrelated to the oral sulfonylurea insulin secretagogues.
Structural Formula of Repaglinide:
Repaglinide is a white to off-white powder with molecular formula C27H36N2O4 and a molecular weight of 452.6. PRANDIN tablets contain 1 mg or 2 mg of repaglinide. In addition, each tablet contains the following inactive ingredients: dicalcium phosphate (anhydrous), microcrystalline cellulose, corn starch, meglumine, croscarmellose sodium, povidone, poloxamer, magnesium stearate, and colloidal silicon dioxide. The 1 mg and 2 mg tablets contain iron oxides (yellow and red, respectively) as coloring agents.
| Dosage Forms and Strengths |
|---|
|
| How Supplied |
|---|
|
PRANDIN (repaglinide) tablets, 1 mg, are supplied as yellow, round, biconvex tablets, debossed with "745" on one side and 'C' on the other side. They are available as follows: Bottles of 100 NDC 60846-882-01 PRANDIN (repaglinide) tablets, 2 mg, are supplied as pink, round, biconvex tablets, debossed with "747" on one side and 'C' on the other side. They are available as follows: Bottles of 100 NDC 60846-884-01 Distributed by: Amneal Specialty, a Division of Amneal Pharmaceuticals LLC, Bridgewater, NJ 08807 |
Drugs
| Drug | Countries | |
|---|---|---|
| PRANDIN | Austria, Estonia, Spain, Croatia, Ireland, Italy, Lithuania, Poland, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.