PRED FORTE Eye drops, suspension Ref.[10913] Active ingredients: Prednisolone
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
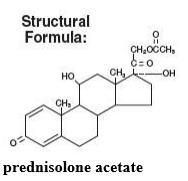
PRED FORTE (prednisolone acetate ophthalmic suspension, USP) 1% is a sterile, topical anti-inflammatory agent for ophthalmic use. Its chemical name is 11ß,17, 21-Trihydroxypregna-1,4-diene-3, 20-dione 21-acetate and it has the following structure:
Each mL of PRED FORTE contains:
Active: prednisolone acetate (microfine suspension) 1%
Inactives: benzalkonium chloride as preservative; boric acid; edetate disodium; hypromellose; polysorbate 80; purified water; sodium bisulfite; sodium chloride; and sodium citrate.
The pH during its shelf life ranges from 5.0-6.0.
| How Supplied |
|---|
|
PRED FORTE (prednisolone acetate ophthalmic suspension, USP) 1% is supplied sterile in opaque white LDPE plastic bottles with droppers with pink high impact polystyrene (HIPS) caps as follows: 1 mL in 5 mL bottle – NDC 11980-180-01 Distributed by: Allergan USA, Inc., Madison, NJ 07940 |
Drugs
| Drug | Countries | |
|---|---|---|
| PRED FORTE | Canada, Spain, Finland, Ireland, Israel, Netherlands, New Zealand, Singapore, Turkey, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.