PREXUM Film-coated tablet Ref.[50301] Active ingredients: Perindopril
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2022 Publisher: Servier Laboratories (Aust.) Pty. Ltd., www.servier.com.au, Level 4, Building 9, 588A Swan Street, Burnley, 3121, Victoria
Product name and form
PREXUM Perindopril arginine.
| Pharmaceutical Form |
|---|
|
PREXUM 2.5 is a white, round, convex, film-coated tablet. PREXUM 5 is a light-green, rod-shaped, film-coated tablet engraved with a "Servier" logo on one face and scored on both edges. PREXUM 10 is a green, round, biconvex film-coated tablet with a "Servier" logo on one face and a "heart" logo on the other face. |
Qualitative and quantitative composition
Each PREXUM 2.5 tablet contains 2.5 mg of perindopril arginine. Each PREXUM 5 tablet contains 5 mg of perindopril arginine. Each PREXUM 10 tablet contains 10 mg of perindopril arginine.
Excipient with known effect: contains sugars as lactose
For the full list of excipients, see section 6.1 - List of excipients.
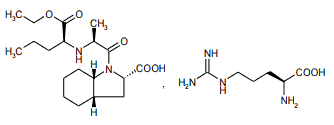
The active component of PREXUM is perindopril arginine which has the chemical name, L-arginine(2S,3aS,7aS)-1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl)butyl]amino] propanoyl]octahydro-1H-indole-2-carboxylate. Perindopril is a dipeptide monoacid monoester with a perhydroindole group and no sulfydryl radical. Perindopril arginine is a white powder, readily soluble in purified water, slightly soluble in 95% ethanol and practically insoluble in chloroform. Perindopril has five asymmetric centres and is synthesised stereoselectively so that it is a single enantiomer (all S stereochemistry).
Chemical structure
CAS number: 612548-45-5
| Active Ingredient |
|---|
|
Perindopril is an inhibitor of the enzyme that converts angiotensin I into angiotensin II (Angiotensin Converting Enzyme ACE). The converting enzyme, or kinase, is an exopeptidase that allows conversion of angiotensin I into the vasoconstrictor angiotensin II as well as causing the degradation of the vasodilator bradykinin into an inactive heptapeptide. Inhibition of ACE results in a reduction of angiotensin II in the plasma, which leads to increased plasma renin activity (by inhibition of the negative feedback of renin release) and reduced secretion of aldosterone. Since ACE inactivates bradykinin, inhibition of ACE also results in an increased activity of circulating and local kallikrein-kinin systems (and thus also activation of the prostaglandin system). |
| List of Excipients |
|---|
|
PREXUM 2.5, PREXUM 5 & PREXUM 10: Hydrophobic colloidal silica anhydrous PREXUM 5 only: Premix for light-green colour coating [copper chlorophyllin (E141ii)] PREXUM 10 only: Premix for green colour coating [copper chlorophyllin (E141ii)] |
Pack sizes and marketing
Thirty (30) tablets supplied in a white HDPE bottle equipped with a white induction-sealed child resistant-closure and desiccant sachets. PREXUM 5 only, is also supplied in a 10-tablet bottle.
Marketing authorization holder
Servier Laboratories (Aust.) Pty. Ltd., www.servier.com.au, Level 4, Building 9, 588A Swan Street, Burnley, 3121, Victoria
Marketing authorization dates and numbers
12 February 2013
Drugs
| Drug | Countries | |
|---|---|---|
| PREXUM | Australia, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.