PRISMASOL Renal replacement solution Ref.[50589] Active ingredients: Calcium chloride Glucose Lactic acid Magnesium chloride Potassium chloride Sodium bicarbonate Sodium chloride
Source: FDA, National Drug Code (US) Revision Year: 2022
1. Indications and Usage
PRISMASOL and PHOXILLUM solutions are indicated in pediatric and adult patients for use as a replacement solution in Continuous Renal Replacement Therapy (CRRT) to replace plasma volume removed by ultrafiltration and to correct electrolyte and acidbase imbalances. They may also be used in case of drug poisoning when CRRT is used to remove dialyzable substances.
2. Dosage and Administration
2.1 Administration Instructions
Visually inspect PRISMASOL and PHOXILLUM for particulate matter and discoloration prior to administration.
Administration should only be under the direction of a physician competent in intensive care treatment including CRRT. Use only with extracorporeal dialysis equipment appropriate for CRRT.
The prepared solution is for single patient use only.
Aseptic technique should be used throughout administration to the patient.
Discard any unused solution.
2.2 Dosing Considerations
PRISMASOL replacement solutions contain 4 different combinations of active ingredients (7 different products with varying ingredient amounts). PHOXILLUM replacement solutions contain 2 different combinations of active ingredients (2 different products with varying ingredient amounts). PRISMASOL and PHOXILLUM are supplied in a twocompartment bag that must be mixed immediately prior to use [see Dosage and Administration (2.3)]:
- Small compartment A (250 mL) containing an electrolyte solution, and
- Large compartment B (4750 mL) containing the buffer solution.
See Table 1 for the concentrations of the active ingredients (after mixing) in these 9 different replacement solutions (total volume is 5 Liters).
Table 1. Concentrations of Active Ingredients in the 7 PRISMASOL and 2 PHOXILLUM Replacement Solutions after Mixing:
| Ca2+ mEq/L | HCO3- mEq/L | K+ mEq/L | Mg2+ mEq/L | Na+ mEq/L | HPO42- mmol/L | Cl- mEq/L | Lactate mEq/L | Dextrose mg/dL | Osmolarity mOsm/L | |
|---|---|---|---|---|---|---|---|---|---|---|
| PRISMASOL Replacement Solutions | ||||||||||
| BGK0/2.5 | 2.5 | 32 | 0 | 1.5 | 140 | 0 | 109 | 3 | 100 | 292 |
| BGK4/2.5 | 2.5 | 32 | 4 | 1.5 | 140 | 0 | 113 | 3 | 100 | 300 |
| BGK2/3.5 | 3.5 | 32 | 2 | 1 | 140 | 0 | 111.5 | 3 | 100 | 296 |
| BGK2/0 | 0 | 32 | 2 | 1 | 140 | 0 | 108 | 3 | 100 | 291 |
| B22GK4/0 | 0 | 22 | 4 | 1.5 | 140 | 0 | 120.5 | 3 | 100 | 296 |
| BGK4/0/1.2 | 0 | 32 | 4 | 1.2 | 140 | 0 | 110.2 | 3 | 100 | 295 |
| BK0/0/1.2 | 0 | 32 | 0 | 1.2 | 140 | 0 | 106.2 | 3 | 0 | 282 |
| PHOXILLUM Replacement Solutions | ||||||||||
| BK4/2.5 | 2.5 | 32 | 4 | 1.5 | 140 | 1 | 114.5 | 0 | 0 | 294 |
| B22K4/0 | 0 | 22 | 4 | 1.5 | 140 | 1 | 122 | 0 | 0 | 290 |
Ca2+ = calcium, HCO3 = bicarbonate, K = potassium, Mg2 = magnesium, Na+ = sodium, HPO42 = phosphate, Cl- = chloride; osmolarity is estimated
The mode of therapy, solute formulation, flow rates, and length of PRISMASOL and PHOXILLUM replacement therapy in CRRT should be established by a physician based on the patient's clinical condition, blood concentration of phosphate and other electrolytes, acid-base and glucose balance. Administer either PRISMASOL or PHOXILLUM into the extracorporeal circuit:
- Before (pre-dilution) the hemofilter or hemodiafilter,
- After (post-dilution) the hemofilter or hemodiafilter, or
- Before and after the hemofilter or hemodiafilter.
2.3 Preparing the Solution
Use only if the overwrap is not damaged, all seals are intact, peel seal is not broken, and the solution is clear.
The solution may be warmed to 37°C/98.6°F prior to removing the overwrap to enhance patient comfort. However, only dry heat should be used. Solutions should not be heated in water or in a microwave oven. After heating, verify that the solution remains clear and contains no particulate matter.
The solutions are supplied in two different two-compartment bags made of polyolefin with a peel seal separating compartment A and B (see Figure 1).
Follow the instructions below when connecting the solution bags for correct use of the access ports.
Instructions for preparing solutions supplied in a two-compartment, polyolefin bag with a peel seal:
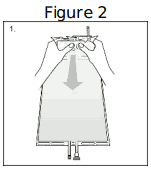 | Step 1. Immediately before use, remove the overwrap from the bag and mix the solutions in the two different compartments. After removing the overwrap, inspect the bag for leakage by pressing firmly on the bag. Discard the bag if any leakage is detected since sterility cannot be assured. As soon as the overwrap is removed, the reconstitution of compartments A and B should be done and the mixed solution should be used immediately. After removal of the overwrap, the solution is stable for 24 hours including the duration of the treatment. Hold the small compartment with both hands and squeeze it until an opening is created in the peel seal. (See Figure 2 beside) |
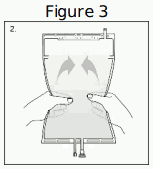 | Step 2. Squeeze with both hands on the large compartment until the peel seal between the two compartments is entirely open. Shake gently to mix. (See Figure 3 beside). The solution is now ready to use and the bag can be hung on the equipment. |
 | Step 3. The replacement line may be connected to the bag through either of the luer connector or the injection connector (spike connector). Step 3a. The luer connector is a needle-less and swabbable connector. Remove the cap with a twist and pull motion, and connect the male luer lock on the replacement line to the female luer receptor on the bag. (See Figure 4a beside). Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely during use. When the replacement line is disconnected from the luer connector, the connector will close and the flow of the solution will stop. |
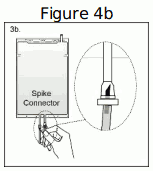 | Step 3b. If the injection connector (spike connector) is used, first remove the snap-off cap. Then introduce the replacement line spike through the rubber septum of the bag connector (See Figure 4b beside) Ensure that the spike is fully inserted and verify that the fluid is flowing freely during use. |
2.4 Adding Drugs to the Solutions
After mixing, additional drugs may be added to the bag via injection connector (spike connector) in large compartment B. In general, administer drugs other than phosphate through a different access line.
When introducing drugs, use aseptic techniques and mix thoroughly prior to connecting the solution bag to the extracorporeal circuit.
Do not use if there is a color change and/or the appearance of precipitates, insoluble complexes or crystals after addition of medication.
Phosphate: Up to 1.2 mmol/L of phosphate can be added to the bag as potassium phosphate or sodium phosphate. The total potassium concentration of PRISMASOL solution should not exceed 4 mEq/L. Use sodium phosphate to add phosphate if the total potassium concentration in PRISMASOL solution is 4 mEq/L.
PHOXILLUM Solutions
Phosphate: Phosphate up to 0.2 mmol/L may be added to the solution. Use sodium phosphate if adding phosphate to bag. The total phosphate concentration should not exceed 1.2 mmol/L.
16.2. Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
Do not freeze or expose to excessive heat. Do not use if precipitate has formed or if container seals have been damaged.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.
