QSYMIA Extended release capsule Ref.[50588] Active ingredients: Phentermine Topiramate
Source: FDA, National Drug Code (US) Revision Year: 2022
1. Indications and Usage
QSYMIA is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in:
- Adults with an initial body mass index (BMI) of:
- 30 kg/m² or greater (obese), or
- 27 kg/m² or greater (overweight) in the presence of at least one weight-related comorbidity such as hypertension, type 2 diabetes mellitus, or dyslipidemia
- Pediatric patients aged 12 years and older with an initial BMI in the 95th percentile or greater standardized for age and sex.
Limitations of Use:
- The effect of QSYMIA on cardiovascular morbidity and mortality has not been established.
- The safety and effectiveness of QSYMIA in combination with other products intended for weight loss, including prescription drugs, over-the-counter drugs, and herbal preparations, have not been established.
2. Dosage and Administration
2.1 Patient Selection
Adults
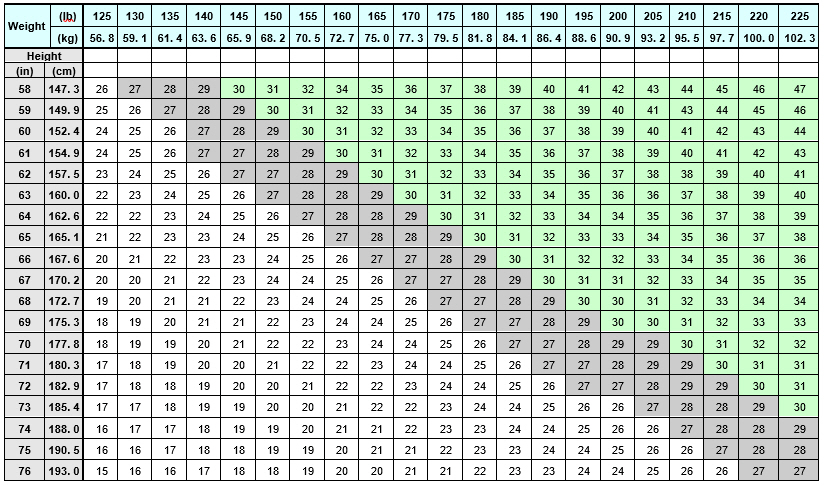
Select adult patients for QSYMIA treatment based on BMI [see Indications and Usage (1)]. Determine the patient's BMI by dividing weight (in kilograms) by height (in meters) squared. A BMI conversion table based on height [inches (in) or centimeters (cm)] and weight [pounds (lb) or kilograms (kg)] is provided below (see Table 1).
Table 1. BMI Conversion Chart:
Pediatric Patients Aged 12 Years and Older
Select pediatric patients for QSYMIA treatment based on BMI percentile [see Indications and Usage (1)]. See Table 2 for BMI percentiles by age and sex for pediatric patients aged 12 years and older.
Table 2. BMI Percentiles by Age and Sex for Pediatric Patients Aged 12 Years and Older:
| Male | Female | |
|---|---|---|
| Age (in years) | 95th Percentile BMI Value | 95th Percentile BMI Value |
| 12 | 24.2 | 25.3 |
| 12.5 | 24.7 | 25.8 |
| 13 | 25.2 | 26.3 |
| 13.5 | 25.6 | 26.8 |
| 14 | 26.0 | 27.3 |
| 14.5 | 26.5 | 27.7 |
| 15 | 26.8 | 28.1 |
| 15.5 | 27.2 | 28.5 |
| 16 | 27.6 | 28.9 |
| 16.5 | 27.9 | 29.3 |
| 17 | 28.3 | 29.6 |
| 17.5 | 28.6 | 30.0 |
2.2 Recommended Testing Prior to and During Treatment with QSYMIA
Prior to QSYMIA initiation and during treatment with QSYMIA, the following is recommended:
- Obtain a negative pregnancy test before initiating QSYMIA in patients who can become pregnant and monthly during QSYMIA therapy. QSYMIA is contraindicated during pregnancy [see Contraindications (4), Warnings and Precautions (5.1) and Use in Specific Populations (8.3)].
- Obtain a blood chemistry profile that includes bicarbonate, creatinine, and potassium in all patients, and glucose in patients with type 2 diabetes on antidiabetic medication prior to initiating QSYMIA treatment and periodically during treatment [Warnings and Precautions (5.8, 5.9, 5.10, 5.15)].
2.3 Recommended Dosage and Administration
The recommended dosage, titration, and administration of QSYMIA are as follows:
- Take QSYMIA orally once daily in the morning with or without food. Avoid administration of QSYMIA in the evening due to the possibility of insomnia.
- The recommended starting dosage is QSYMIA 3.75 mg/23 mg (phentermine 3.75 mg/topiramate 23 mg) orally once daily for 14 days; after 14 days increase to the recommended dosage of QSYMIA 7.5 mg/46 mg (phentermine 7.5 mg/topiramate 46 mg) orally once daily.
- After 12 weeks of treatment with QSYMIA 7.5 mg/46 mg, evaluate weight loss for adults or BMI reduction for pediatric patients aged 12 years and older. If an adult patient has not lost at least 3% of baseline body weight or a pediatric patient has not experienced a reduction of at least 3% of baseline BMI, increase the dosage to QSYMIA 11.25 mg/69 mg (phentermine 11.25 mg/topiramate 69 mg) orally once daily for 14 days; followed by an increase in the dosage to QSYMIA 15 mg/92 mg (phentermine 15 mg/topiramate 92 mg) orally once daily.
- After 12 weeks of treatment with QSYMIA 15 mg/92 mg, evaluate weight loss for adults or BMI reduction for pediatric patients aged 12 years and older. If an adult patient has not lost at least 5% of baseline body weight or a pediatric patient has not experienced a reduction of at least 5% of baseline BMI, discontinue QSYMIA [see Dosage and Administration (2.4)], as it is unlikely that the patient will achieve and sustain clinically meaningful weight loss with continued treatment.
- Monitor the rate of weight loss in pediatric patients. If weight loss exceeds 2 lbs (0.9 kg)/week, consider dosage reduction.
2.4 Discontinuation of QSYMIA 15 mg/92 mg
Discontinue QSYMIA 15 mg/92 mg gradually by taking QSYMIA 15 mg/92 mg once daily every other day for at least 1 week prior to stopping treatment altogether, due to the possibility of precipitating a seizure [see Warnings and Precautions (5.12) and Drug Abuse and Dependence (9.3)].
2.5 Recommended Dosage in Patients with Renal Impairment
Avoid use of QSYMIA in patients with end-stage renal disease on dialysis.
In patients with severe (creatinine clearance [CrCl] less than 30 mL/min) or moderate (CrCl greater than or equal to 30 and less than 50 mL/min) renal impairment (CrCl calculated using the Cockcroft-Gault equation with actual body weight), the maximum recommended dosage is QSYMIA 7.5 mg/46 mg once daily.
The recommended dosage in patients with mild (CrCl greater or equal to 50 and less than 80 mL/min) renal impairment is the same as the recommended dosage for patients with normal renal function [see Warnings and Precautions (5.9), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
2.6 Recommended Dosage in Patients with Hepatic Impairment
Avoid use of QSYMIA in patients with severe hepatic impairment (Child-Pugh score 10-15). In patients with moderate hepatic impairment (Child-Pugh score 7-9), the maximum recommended dosage is QSYMIA 7.5 mg/46 mg once daily.
The recommended dosage of QSYMIA in patients with mild hepatic impairment (Child-Pugh 5-6) is the same as the recommended dosage in patients with normal hepatic function [see Use in Specific Populations (8.7), and Clinical Pharmacology (12.3)].
10. Overdosage
In the event of a significant overdose with QSYMIA, if the ingestion is recent, the stomach should be emptied immediately by gastric lavage or by induction of emesis. Appropriate supportive treatment should be provided according to the patient's clinical signs and symptoms.
Acute overdose of phentermine may be associated with restlessness, tremor, hyperreflexia, rapid respiration, confusion, aggressiveness, hallucinations, and panic states. Fatigue and depression usually follow the central stimulation. Cardiovascular effects include arrhythmia, hypertension or hypotension, and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea, and abdominal cramps. Fatal poisoning usually terminates in convulsions and coma. Manifestations of chronic intoxication with anorectic drugs include severe dermatoses, marked insomnia, irritability, hyperactivity, and personality changes. A severe manifestation of chronic intoxication is psychosis, often clinically indistinguishable from schizophrenia.
Management of acute phentermine intoxication is largely symptomatic and includes lavage and sedation with a barbiturate. Acidification of the urine increases phentermine excretion. Intravenous phentolamine has been suggested for possible acute, severe hypertension, if this complicates phentermine overdosage.
Topiramate overdose has resulted in severe metabolic acidosis. Other signs and symptoms include convulsions, drowsiness, speech disturbance, blurred vision, diplopia, impaired mentation, lethargy, abnormal coordination, stupor, hypotension, abdominal pain, agitation, dizziness, and depression. The clinical consequences were not severe in most cases, but deaths have been reported after overdoses involving topiramate. A patient who ingested a dose between 96 and 110 gm topiramate was admitted to hospital with coma lasting 20 to 24 hours followed by full recovery after 3 to 4 days.
Hemodialysis is an effective means of removing topiramate from the body.
16.2. Storage and Handling
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Keep container tightly closed and protect from moisture.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.