RADICAVA Solution for injection Ref.[10273] Active ingredients: Edaravone
Source: FDA, National Drug Code (US) Revision Year: 2018
Product description
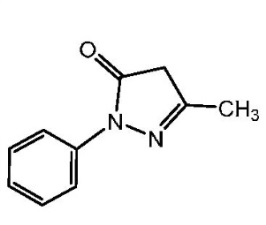
The active ingredient in RADICAVA is edaravone, which is a member of the substituted 2-pyrazolin-5-one class. The chemical name of edaravone is [3-methyl-1-phenyl-2-pyrazolin-5-one]. The molecular formula is C10H10N2O and the molecular weight is 174.20.
The chemical structure is:
Edaravone is a white crystalline powder with a melting point of 129.7°C. It is freely soluble in acetic acid, methanol, or ethanol and slightly soluble in water or diethyl ether.
RADICAVA injection is a clear, colorless liquid provided as a sterile solution.
RADICAVA injection is supplied for intravenous infusion in a polypropylene bag containing 30 mg edaravone in 100 mL isotonic, sterile, aqueous solution, which is further overwrapped with polyvinyl alcohol (PVA) secondary packaging. The overwrapped package also contains an oxygen absorber and oxygen indicator to minimize oxidation. Each bag contains the following inactive ingredients: L-cysteine hydrochloride hydrate (10 mg), sodium bisulfite (20 mg). Sodium chloride is added for isotonicity and phosphoric acid and sodium hydroxide are added to adjust to pH 4.
| Dosage Forms and Strengths |
|---|
|
RADICAVA is supplied for intravenous infusion in a single-dose polypropylene bag containing 30 mg of edaravone in 100 mL of clear, colorless aqueous solution. |
| How Supplied |
|---|
|
RADICAVA injection is supplied as a 30 mg/100 mL (0.3 mg/mL) clear, colorless, sterile solution for intravenous infusion in single-dose polypropylene bags, each overwrapped with polyvinyl alcohol (PVA) secondary packaging containing an oxygen absorber and oxygen indicator, which should be pink to reflect appropriate oxygen levels [see Dosage and Administration (2.2) and How Supplied/Storage and Handling(16.2)]. These are supplied in cartons as listed below.
|
Drugs
| Drug | Countries | |
|---|---|---|
| RADICAVA | Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.