REMOVAB Concentrate for solution for infusion Ref.[27988] Active ingredients: Catumaxomab
Source: European Medicines Agency (EU) Revision Year: 2017 Publisher: Neovii Biotech GmbH, Am Haag 6-7, 82166 Graefelfing, Germany
4.1. Therapeutic indications
Removab is indicated for the intraperitoneal treatment of malignant ascites in adults with EpCAM-positive carcinomas where standard therapy is not available or no longer feasible.
4.2. Posology and method of administration
Removab must be administered under the supervision of a physician experienced in the use of anti-neoplastic medicinal products.
Posology
Prior to the intraperitoneal infusion, pre-medication with analgesic/antipyretic/non-steroidal antiphlogistic medicinal products is recommended (see section 4.4).
Removab dosing schedule comprises the following four intraperitoneal infusions:
1st dose 10 micrograms on day 0
2nd dose 20 micrograms on day 3
3rd dose 50 micrograms on day 7
4th dose 150 micrograms on day 10
Removab has to be administered as constant rate intraperitoneal infusion with an infusion time of at least 3 hours. In clinical studies infusion times of 3 hours and 6 hours were investigated. For the first of the four doses an infusion time of 6 hours may be considered depending on the patient’s health condition.
An interval of at least two infusion free calendar days must elapse between infusion days. The interval between the infusion days can be prolonged in case of relevant adverse reactions. The overall treatment period should not exceed 20 days.
Monitoring
Adequate monitoring of the patient after end of Removab infusion is recommended. In the pivotal study patients were monitored for 24 h after each infusion.
Special populations
Hepatic impairment
Patients with hepatic impairment of a higher severity grade than moderate and/or with more than 70% of the liver metastasised and/or portal vein thrombosis/obstruction have not been investigated. Treatment of these patients with Removab should only be considered after a thorough evaluation of benefit/risk (see section 4.4).
Renal impairment
Patients with renal impairment of a higher severity grade than mild have not been investigated. Treatment of these patients with Removab should only be considered after a thorough evaluation of benefit/risk (see section 4.4).
Paediatric population
There is no relevant use of Removab in the paediatric population in the granted indication.
Method of administration
Removab must be administered as an intraperitoneal infusion only.
Removab must not be administered by intraperitoneal bolus or by any other route of administration.
For information on the perfusion system to be used see section 4.4.
Precautions to be taken before administering the medicinal product
Before administration of Removab the concentrate for solution for infusion is diluted in sodium chloride 9 mg/ml (0.9%) solution for injection. The diluted Removab solution for infusion is administered intraperitoneally as constant rate infusion using an adequate pump system.
For instructions on dilution of the medicinal product before administration, see section 6.6.
4.9. Overdose
No case of overdose has been reported. Patients receiving a higher than recommended dose of catumaxomab experienced more severe (grade 3) adverse reactions.
6.3. Shelf life
2 years.
After dilution:
The prepared solution for infusion is physically and chemically stable for 48 hours at 2°C to 8°C and for 24 hours at a temperature not above 25°C. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2°C to 8°C, unless dilution has taken place in controlled and validated aseptic conditions.
6.4. Special precautions for storage
Store in a refrigerator (2°C-8°C). Do not freeze. Store in the original package in order to protect from light.
For storage conditions after dilution of the medicinal product, see section 6.3.
6.5. Nature and contents of container
0.1 ml concentrate for solution for infusion in a pre-filled syringe (type I glass, siliconised) with plunger stopper (bromobutyl rubber) and luer lock system (polypropylene siliconised and polycarbonate) with tip cap (styrene butadiene rubber) with a cannula; pack size of 1.
6.6. Special precautions for disposal and other handling
Disposal
No special requirements.
Material and equipment required
The following components must be used for the dilution and administration of Removab as Removab is only compatible with:
- 50 ml polypropylene syringes
- polyethylene perfusion tubings with an inner diameter of 1 mm and a length of 150 cm
- polycarbonate infusion valves / Y connections
- polyurethane, polyurethane silicon coated catheters
In addition the following is required:
- Sodium chloride 9 mg/ml (0.9%) solution for injection
- Precision perfusion pump
Instructions for dilution prior to administration
Removab should be prepared by a healthcare professional using appropriate aseptic technique. The outer surface of the pre-filled syringe is not sterile.
- Based on the dose, the appropriate amount of sodium chloride 9 mg/ml (0.9%) solution for injection is extracted with a 50 ml syringe (Table 7).
- An additional air buffer of at least 3 ml is included in the 50 ml syringe.
- The tip cap from the Removab pre-filled syringe is removed with the tip pointing up.
- The enclosed cannula is attached to the Removab pre-filled syringe. For each syringe a new cannula is used.
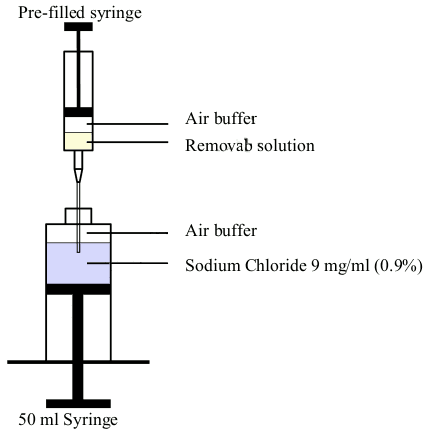
- The pre-filled syringe cannula is inserted through the 50 ml syringe opening so that the cannula is immersed in the sodium chloride 9 mg/ml (0.9%) solution for injection (Figure 2).
- The entire content of the syringe (Removab concentrate plus air buffer) is injected from the pre-filled syringe directly into the sodium chloride 9 mg/ml (0.9%) solution for injection.
- The plunger rod MUST NOT be drawn back to rinse the pre-filled syringe, in order to avoid contamination and to ensure that the correct volume is ejected.
- The 50 ml syringe is closed with a cap and shaken gently to mix the solution. Any air bubble(s) from the 50 ml syringe is eliminated.
- The peelable sticker, which is provided on the inner side of the Removab carton box, displaying the text “Diluted Removab. Intraperitoneal use only.” must be attached to the 50 ml syringe containing the diluted Removab solution for intraperitoneal infusion. This is a precautionary measure to ensure that Removab is infused only via the intraperitoneal route of administration.
- The 50 ml syringe is inserted in the infusion pump.
Table 7. Preparation of Removab solution for intraperitoneal infusion:
| Number of infusion / Dose | Number of Removab pre-filled syringe(s) | Total volume of Removab concentrate for solution for infusion | Sodium chloride 9 mg/ml (0.9%) solution for injection | Final volume for administration | |
|---|---|---|---|---|---|
| 10 micrograms pre-filled syringe | 50 micrograms pre-filled syringe | ||||
| 1th infusion 10 micrograms | 1 | 0.1 ml | 10 ml | 10.1 ml | |
| 2th infusion 20 micrograms | 2 | 0.2 ml | 20 ml | 20.2 ml | |
| 3th infusion 50 micrograms | 1 | 0.5 ml | 49.5 ml | 50 ml | |
| 4th infusion 150 micrograms | 3 | 1.5 ml | 48.5 ml | 50 ml | |
Figure 2. Illustration of the transfer of Removab from the pre-filled syringe to the 50 ml syringe:
Method of administration
The catheter for intraperitoneal administration should be placed under ultrasound guidance by a physician experienced in intraperitoneal administration procedures. The catheter is used for ascites drainage and infusion of diluted Removab and sodium chloride 9 mg/ml (0.9%) solution for injection. It is recommended that the catheter remains in the abdominal cavity during the entire treatment period. It can be removed the day after the last infusion.
Prior to each Removab administration the ascites fluid must be drained until stop of spontaneous flow or symptom relief (see section 4.4). Subsequently, prior to each Removab administration 500 ml sodium chloride 9 mg/ml (0.9%) solution for injection shall be infused to support distribution of the antibody in the abdominal cavity.
Removab must be administered intraperitoneally over an infusion time of at least 3 hours via a constant infusion pump system as described below:
- The 50 ml syringe containing the diluted Removab solution for infusion is installed in the precision pump.
- The connected perfusion tubing equipment of the precision pump is prefilled with the diluted Removab solution for infusion. A perfusion tubing of an inner diameter of 1 mm and a length of 150 cm must be used.
- The perfusion tubing is connected to the Y-connection.
- Parallel to each Removab application 250 ml sodium chloride 9 mg/ml (0.9%) solution for injection are infused via an infusion valve / Y connection in the perfusion lead of the catheter.
- The pump speed is adjusted according to the volume to be administered and the scheduled infusion time.
- When the 50 ml syringe containing the diluted Removab solution for infusion is empty it is replaced with a 50 ml syringe containing 20 ml sodium chloride 9 mg/ml (0.9%) solution for injection until the end of the scheduled infusion time to clear the dead volume in the perfusion lead (approximately 2 ml) under unchanged conditions. The remaining sodium chloride 9 mg/ml (0.9%) solution for injection can be discarded.
- The catheter is kept closed until the next infusion.
- The day after the last infusion a drainage of ascites until stop of spontaneous flow is performed. Subsequently, the catheter can be removed.
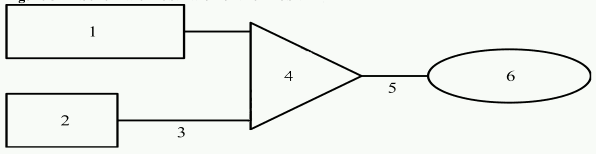
Figure 3. Schematic illustration of the infusion system:
- 250 ml Sodium Chloride 9 mg/ml (0.9%)
- Removab solution for i.p. infusion
- Perfusion Tubing (1 mm inner diameter, 150 cm length)
- Infusion valve
- Perfusion Lead
- Catheter
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.