RESCULA Ophthalmic solution Ref.[50225] Active ingredients: Unoprostone
Source: FDA, National Drug Code (US)
Product description
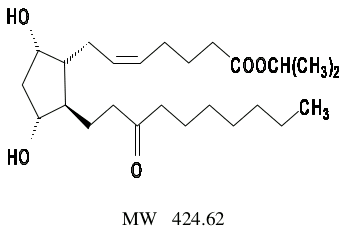
Unoprostone Isopropyl is a docosanoid, a structural analogue of an inactive biosynthetic cyclic derivative of arachidonic acid, 13, 14-dihydro-15-keto-prostaglandin F2α. Its chemical name is isopropyl (+)-7[(1R, 2R, 3R, 5S)-3,5-dihydroxy-2-(3-oxodecyl) cyclopentyl]-5-heptenoate. Its molecular formula is C25H44O5 and its chemical structure is:
Unoprostone isopropyl is a clear, colorless viscous liquid that is very soluble in acetonitrile, ethanol, ethyl acetate, isopropanol, dioxane, ether, and hexane. It is practically insoluble in water. RESCULA (unoprostone isopropyl ophthalmic solution) 0.15% is supplied as a sterile, isotonic, buffered aqueous solution of unoprostone isopropyl with a pH of 5.0–6.5 and an osmolality of 235-300 mOsmol/kg.
Each mL of Rescula contains 1.5 mg of unoprostone isopropyl. Benzalkonium chloride 0.015% is added as a preservative. Inactive ingredients are: mannitol, polysorbate 80, edetate disodium, sodium hydroxide or hydrochloric acid (to adjust pH), and water for injection.
| How Supplied |
|---|
|
RESCULA (unoprostone isopropyl ophthalmic solution) 0.15% is a clear, isotonic, buffered, preserved colorless solution of unoprostone isopropyl 0.15% (1.5 mg/mL). RESCULA 0.15% is supplied as 5 mL solution in a 7.5 mL natural polypropylene bottle with a natural polypropylene dropper tip, a turquoise polypropylene closure and a clear tamper-evident shrinkband. Made in Canada by CIBA Vision Sterile Manufacturing for: CIBA Vision, A Novartis Company, Duluth, GA 30097 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.