RESPREEZA Powder and solvent for solution Ref.[6143] Active ingredients: Alfa1 antitrypsin
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: CSL Behring GmbH, Emil-von-Behring-Strasse 76, D-35041, Marburg, Germany
Therapeutic indications
Respreeza is indicated for maintenance treatment, to slow the progression of emphysema in adults with documented severe alpha1-proteinase inhibitor deficiency (e.g. genotypes PiZZ, PiZ(null), Pi(null,null), PiSZ). Patients are to be under optimal pharmacologic and non-pharmacologic treatment and show evidence of progressive lung disease (e.g. lower forced expiratory volume per second (FEV1) predicted, impaired walking capacity or increased number of exacerbations) as evaluated by a healthcare professional experienced in the treatment of alpha1-proteinase inhibitor deficiency.
Posology and method of administration
First infusions should be administered under the supervision of a healthcare professional experienced in the treatment of alpha1-proteinase inhibitor deficiency. Subsequent infusions can be administered by a caregiver or by the patient (see section 4.4).
Posology
The recommended dose of Respreeza is 60 mg/kg body weight (bw) administered once weekly.
Paediatric population
The safety and efficacy of Respreeza in the paediatric population (below 18 years) have not been established. No data are available.
Elderly population
The safety and efficacy of Respreeza in elderly patients (65 years of age or older) have not been established in specific clinical trials.
Patients with renal or hepatic impairment
No special investigations have been performed. No alternative dose regimen can be recommended in those patients.
Method of administration
Respreeza should only be administered intravenously by infusion after reconstitution. The powder must be reconstituted with water for injections (see instructions on reconstitution in section 6.6) and administered using an intravenous administration set (not supplied).
The reconstituted solution should be infused intravenously using a separate dedicated infusion line at an infusion rate of about 0.08 ml/kg bw/min. This infusion rate may be adjusted, based upon patient tolerability. The recommended dose of 60 mg/kg bw will take approximately 15 minutes to infuse. One vial of Respreeza is for single use only.
For detailed information regarding the administration of the reconstituted solution, see the instructions at the end of section 6.6.
Overdose
Consequences of overdose are unknown.
In the event of overdose, the patient should be observed closely for the occurrence of adverse reactions and supportive measures should be available as necessary.
Shelf life
Respreeza 1,000 mg powder and solvent for solution for infusion: 3 years.
Respreeza 4,000 mg powder and solvent for solution for infusion: 2 years.
Respreeza 5,000 mg powder and solvent for solution for infusion: 2 years.
From a microbiological point of view, the product should be used immediately after reconstitution. However chemical and physical in-use stability has been demonstrated for 3h at room temperature (up to 25°C). Do not freeze the reconstituted solution.
Special precautions for storage
Do not store above 25°C. Do not freeze.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
Nature and contents of container
Respreeza 1,000 mg powder and solvent for solution for infusion: Respreeza 1,000 mg of powder in a glass vial (type I), closed with a rubber (bromobutyl or chlorobutyl) stopper and an aluminium seal with a plastic flip off cap. 20 ml of water for injections in a glass vial (type I), closed with a rubber (chlorobutyl) stopper and an aluminium seal with a plastic flip off cap.
Respreeza 4,000 mg powder and solvent for solution for infusion: Respreeza 4,000 mg of powder in a glass vial (type I), closed with a rubber (bromobutyl or chlorobutyl) stopper and an aluminium seal with a plastic flip off cap. 76 ml of water for injections in a glass vial (type I), closed with a rubber (chlorobutyl) stopper and an aluminium seal with a plastic flip off cap.
Respreeza 5,000 mg powder and solvent for solution for infusion: Respreeza 5,000 mg of powder in a glass vial (type I), closed with a rubber (bromobutyl or chlorobutyl) stopper and an aluminium seal with a plastic flip off cap. 95 ml of water for injections in a glass vial (type I), closed with a rubber (chlorobutyl) stopper and an aluminium seal with a plastic flip off cap.
Presentations
Each pack contains:
Respreeza 1,000 mg powder and solvent for solution for infusion:
One single-use powder vial.
One solvent vial of 20°ml water for injections.
One transfer set 20/20 (Mix2Vial set) for reconstitution.
Respreeza 4,000 mg powder and solvent for solution for infusion:
One single-use powder vial.
One solvent vial of 76°ml water for injections.
One transfer set 20/20 (Mix2Vial set) for reconstitution.
Respreeza 5,000 mg powder and solvent for solution for infusion:
One single-use powder vial.
One solvent vial of 95°ml water for injections.
One transfer set 20/20 (Mix2Vial set) for reconstitution.
Special precautions for disposal and other handling
General instructions
The reconstitution should be performed according to the instructions provided below.
The product must be reconstituted, administered and handled with caution using aseptic technique to maintain product sterility.
Do not use provided sterile ancillaries for reconstitution if their package is opened or if they are damaged.
Inspect the reconstituted solution for particulate matter and discoloration prior to administration.
The reconstituted solution should be clear, colorless to slightly yellow, and free from visible particles.
The powder must be reconstituted with solvent (water for injections).
Total reconstitution of the powder should be obtained within 5 minutes (1 g presentation) or 10 minutes (4 g and 5 g presentation).
Follow the steps provided below for the preparation and reconstitution of Respreeza:
1. Ensure that the Respreeza vial and water for injections vial are at room temperature (up to 25°C).
2. Remove the plastic flip-top cap from the water for injections vial.
3. Wipe the rubber stopper of the water for injections vial with antiseptic solution and allow it to dry.
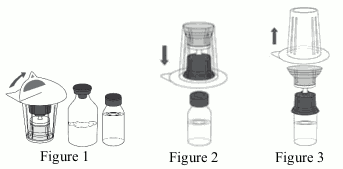
4. Open the Mix2Vial set by peeling off the lid (Figure 1). Do not remove the Mix2Vial set from the blister package.
5. Place the water for injections vial on an even, clean surface and hold the vial tight. Take the Mix2Vial set together with the blister package and vertically pierce the water for injections vial with the blue tip of the Mix2Vial set (Figure 2).
6. Carefully remove the blister package from the Mix2Vial set by holding at the rim, and pulling vertically upwards. Make sure that you only pull away the blister package and not the Mix2Vial set (Figure 3).
7. Remove the plastic flip-top cap from the Respreeza vial.
8. Wipe the rubber stopper of the Respreeza vial with antiseptic solution and allow it to dry
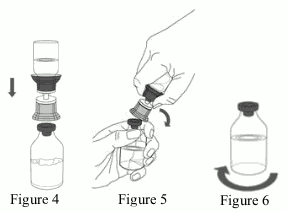
9. Place the Respreeza vial on an even and firm surface. Invert the water for injections vial with the Mix2Vial set attached and vertically pierce the Respreeza vial with the clear tip of the Mix2Vial set (Figure 4). The water for injections will automatically flow into the Respreeza vial.
NOTE: Ensure all water has transferred into the Respreeza vial.
10. Follow steps below to remove entire Mix2Vial set from Respreeza vial:
- With one hand tightly grasp the Respreeza vial as shown in Figure 5.
- With the other hand tightly grasp the water for injections vial and the blue part of the Mix2Vial set.
- Bend the entire Mix2Vial set to the side until it disconnects from the Respreeza vial (Figure 5).
Discard the water for injections vial with the entire Mix2Vial set.
11. Gently swirl the Respreeza vial until the powder is completely dissolved (Figure 6). DO NOT SHAKE. Take care not to touch the rubber vial stopper. Figure 6
12. Inspect visually the reconstituted solution. The solution should be clear, colourless to slightly yellow, and free from visible particles. Do not use solutions that are discoloured, cloudy or have particles.
13. If more than 1 vial of Respreeza is needed to achieve the required dose, repeat instructions 1 to 11 above using an additional package containing an unused Mix2Vial set.
Use a separate, unused Mix2Vial set, and a water for injections vial for each Respreeza vial.
14. Use aseptic technique to transfer the reconstituted solutions into the administration container (e.g., empty intravenous bag or glass bottle; (not supplied) via a commercially available intravenous fluid tubing transfer set (not supplied)).
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.