RESTASIS Emulsion Ref.[50488] Active ingredients: Ciclosporin
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
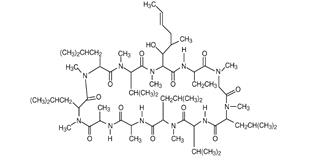
RESTASIS (cyclosporine ophthalmic emulsion) 0.05% contains a topical calcineurin inhibitor immunosuppressant with anti-inflammatory effects. Cyclosporine's chemical name is Cyclo[[(E)-(2S,3R,4R)-3-hydroxy-4-methyl-2-(methylamino)-6-octenoyl]-L-2-aminobutyryl-NmethylglycylNmethyl-L-leucyl-L-valylNmethyl-L-leucyl-L-alanyl-D-alanylNmethyl-L-leucylNmethyl-L-leucylN-methyl-L-valyl] and it has the following structure:
Structural Formula
Formula: C62H111N11O12
Mol. Wt.: 1202.6
Cyclosporine is a fine white powder. RESTASIS appears as a white opaque to slightly translucent homogeneous emulsion. It has an osmolality of 230 to 320 mOsmol/kg and a pH of 6.5-8.0.
Each mL of RESTASIS ophthalmic emulsion contains:
Active: cyclosporine 0.05%.
Inactives: glycerin; castor oil; polysorbate 80; carbomer copolymer type A; purified water; and sodium hydroxide to adjust pH.
| Dosage Forms and Strengths |
|---|
|
Ophthalmic emulsion containing cyclosporine 0.5 mg/mL. |
| How Supplied |
|---|
|
Product: 50090-1242 NDC: 50090-1242-0 .4 mL in a VIAL, SINGLE-USE / 30 in a TRAY Irvine, CA 92612, Made in the U.S.A. |
Drugs
| Drug | Countries | |
|---|---|---|
| RESTASIS | Brazil, Canada, Ecuador, Hong Kong, Israel, Mexico, Turkey, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.