RIBOMUSTIN Powder for solution for infusion Ref.[50298] Active ingredients: Bendamustine
Source: Marketing Authorisation Holder Revision Year: 2022 Publisher: Janssen-Cilag (New Zealand) Ltd, Auckland, NEW ZEALAND, Telephone: 0800 800 806, Fax: (09) 588 1398, Email: medinfo@janau.jnj.com
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, alkylating agents
ATC code: L01AA09
Mechanism of Action
Bendamustine hydrochloride is an alkylating antitumour agent with unique activity. The antineoplastic and cytocidal effect of bendamustine hydrochloride is based essentially on a cross-linking of DNA single and double strands by alkylation. As a result, DNA matrix functions and DNA synthesis and repair are impaired. Bendamustine is active against both quiescent and dividing cells.
The active substance revealed no or very low cross-resistance in human tumour cell lines with different resistance mechanisms.
The exact mechanism of action of bendamustine remains unknown.
Clinical trials
Chronic Lymphocytic Lymphoma
The safety and efficacy of RIBOMUSTIN were evaluated in an open-label, randomised, controlled multicenter trial (02CLLIII) comparing RIBOMUSTIN to chlorambucil. The trial was conducted in 319 previously-untreated patients with Binet Stage B or C CLL requiring treatment. Need-to-treat criteria included hematopoietic insufficiency, B-symptoms, rapidly progressive disease or risk of complications from bulky lymphadenopathy. Patients with autoimmune hemolytic anemia or autoimmune thrombocytopaenia, Richter's syndrome, or transformation to prolymphocytic leukemia were excluded from the study.
The patient populations in the RIBOMUSTIN and chlorambucil treatment groups were balanced with regard to the following baseline characteristics: age (median 63 vs. 66 years), gender (63% vs. 61% male), Binet stage (72% vs. 71% Binet B), lymphadenopathy (79% vs. 82%), enlarged spleen (77% vs. 78%), enlarged liver (49% vs. 45%), hypercellular bone marrow (80% vs. 73%), lymphocyte count (mean 68.9 x 109/L vs. 62.4 x 109/L). Ninety percent of patients in both treatment groups had immuno-phenotypic confirmation of CLL (CD5, CD23 and either CD19 or CD20 or both).
Patients were randomly assigned to receive either RIBOMUSTIN at 100 mg/m², administered intravenously over a period of 30 minutes on Days 1 and 2 or chlorambucil at 0.8 mg/kg (Broca's normal weight) administered orally on Days 1 and 15 of each 28-day cycle.
Efficacy endpoints of objective response rate and progression-free survival were calculated using a pre-specified algorithm based on NCI working group criteria for CLL.
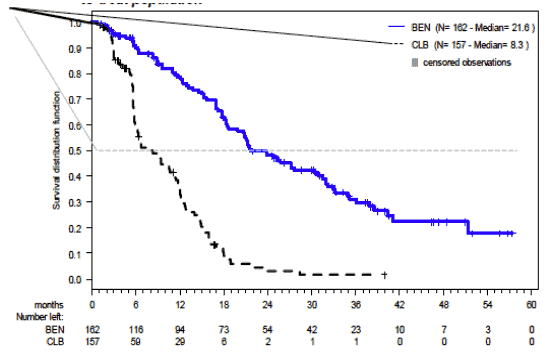
The results of this open-label randomised study demonstrated a higher rate of overall response and a longer progression-free survival for RIBOMUSTIN compared to chlorambucil (see Table 10). Survival data are not mature.
Table 10. Efficacy data for CLL reported in study 02CLLIII:
| RIBOMUSTIN (N=162) | Chlorambucil (N=157) | p-value | |
|---|---|---|---|
| Response Rate n(%) | |||
| Overall response rate | 110 (67.9%) | 48 (30.6%) | <0.0001 |
| (95% CI) | (51.0, 66.6) | (18.6, 32.7) | (64.3, 82.3) |
| Complete response (CR)* | 50 (30.9) | 3 (1.9) | |
| Nodular partial response (nPR)** | 17 (10.5) | 4 (2.5) | |
| Partial response (PR)† | 43 (26.5%) | 41 (26.1%) | |
| Progression-Free Survival†† | |||
| Median, month (95% CI) | 21.6 (11.7, 23.5) | 8.3 (5.6, 8.6) | |
| Hazard ratio (95% CI) | 0.27 (0.17, 0.43) | ||
CI = confidence interval
* CLL response was valued as CR when all of the following criteria were met for at least 8 weeks after first response was observed:
* Enlarged lymph nodes are no longer detectable by palpation (X-ray or ultrasound are optional);
* Absence of hepatomegaly or splenomegaly, confirmed by palpation. CT and ultrasound were optional;
* No disease symptoms (B-symptoms);
* Blood counts:
** Lymohocytes ≤4.0 x 109/L
** Neutrophils ≥1.5 x 109/L
** Platelets >100 x 109/L
** Hemoglobin > 11 g/dL (without blood transfusion)
* Bone marrow biopsy (histology and cytology) was to be performed 8 weeks after meeting the above criteria. The bone marrow had to be at least normocellular for age, with less than 30% lymphocytes.
** nPR was defined as described for CR with lymphocyte being less than 30% in the bone marrow sample but still showing focal infiltration.
† PR was defined as ≥50% decrease in peripheral blood lymphocyte count from the pretreatment baseline value, and either ≥50% reduction of enlarged lymph nodes (total of affected lymph nodes), or 50% reduction of hepatomegaly and/or spelomegaly, as well as one of the following haematologic improvements: neutrophils ≥ 1.5 x 109/L or 50% improvement over baseline, platelets >100 x 109/L or 50% improvement over baseline, haemoglobin >11g/dL or 50% improvement over baseline without transfusions, for a period of at least 8 weeks.
†† PFS was defined as time from randomization to progression or relapse after inter-current remission or death from any cause.
Progression free survival based upon the Independent Committee Response Assessment (ICRA) criteria by treatment group in the follow-up report (ITT analysis) in Study 02CLLIII is shown in Figure 1.
Figure 1. Progression-Free Survival:
Previously Untreated Advanced Indolent Non-Hodgkin's Lymphoma (NHL) and Mantle Cell Lymphoma (MCL)
The safety and efficacy of RIBOMUSTIN in previously untreated advanced indolent NHL and MCL have been assessed in a Phase III trial.
The NHL1-2003 study is a prospective phase III, multicentre, randomised (1:1), non-inferiority, open-label clinical study of 549 patients, conducted to determine that RIBOMUSTIN (90 mg/m²) in combination with rituximab 375 mg/m² is non-inferior to CHOP (cycles every 3 weeks of cyclophosphamide 750 mg/m², doxorubicin 50 mg/m², and vincristine 1.4 mg/m² on day 1, and prednisone 100 mg/day for 5 days) plus rituximab 375 mg/m². Rituximab was administered in both treatment arms on day 1 of each cycle. Treatment was administered for a maximum of 6 cycles. Baseline demographics and patient characteristics are summarized in Table 11.
Table 11. Summary of Baseline Patient and Disease Characteristics in the NHL1-2003 Study:
| Patient Characteristics | B-R N=261 | CHOP-R N=253 | |||
|---|---|---|---|---|---|
| Age (years) | 64 (34-83) | 63 (31-82) | |||
| <60 | 94 (63%) | 90 (36%) | |||
| 61-70 | 107 (41%) | 105 (42%) | |||
| >70 | 60 (23%) | 58 (23%) | |||
| Stage | |||||
| II | 9 (3%) | 9 (4%) | |||
| III | 50 (19%) | 47 (19%) | |||
| IV | 202 (77%) | 197 (78%) | |||
| Histology | |||||
| Follicular | 139 (53%) | 140 (55%) | |||
| Mantle cell | 46 (18%) | 48 (19%) | |||
| Marginal zone | 37 (14%) | 30 (12%) | Lymphoplasmacytic* | 22 (9%) | 19 (8%) |
| Small lymphocytic | 10 (4%) | 11 (4%) | |||
| Low grade, unclassifiable | 7 (3%) | 5 (2%) | |||
| B symptoms | 100 (38%) | 74 (29%) | |||
| Bone marrow involved | 177 (68%) | 170 (67%) | |||
| Extranodal involved sites ≥1 | 212 (81%) | 193 (76%) | |||
| LDH >240 U/L | 100 (38%) | 84 (33%) | |||
| Median ß-2 microglobulin (mg/L) | 2.6 (0.7-17.8) | 2.4 (1.1-23.2) | |||
| Prognostic groups for all patients (IPI) | |||||
| >2 risk factors | 96 (37%) | 89 (35%) | |||
| Prognostic groups according to FLIPI | |||||
| Low risk (0-1 risk factor) | 16/139 (12%) | 26/140 (19%) | |||
| Intermediate risk (2 risk factors) | 5/139 (41%) | 44/140 (31%) | |||
| Poor risk (3-5 risk factors) | 63/136 (46%) | 64/134 (48%) | |||
Data are median (range), n (%), or n/N (%).
B-R=bendamustine plus rituximab; R-CHOP=CHOP plus rituximab; LDH=lactate dehyrogenase; IPI=International Prognostic Index; FLIPI-Folicular Lymphoma International Prognostic Index.
* Waldenström macroglobulinaemia.
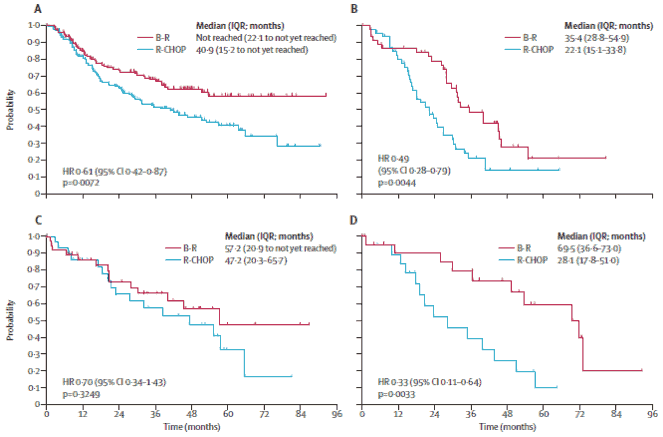
A significant benefit for progression-free survival was shown with B-R vs. R-CHOP for all histological subtypes except for marginal-zone lymphoma (see Figure 2).
Figure 2: Progression-free survival in histological subtypes of follicular lymphoma (A), mantlecell lymphoma (B), marginal-zone lymphoma (C), and Walderstrom's macrglobulinaemia (D):
B-R=bendamustine plus rituximab; R-CHOP=Chop plus rituximab
The improvement in progression-free survival with B-R was independent of age, concentration of lactate dehydrohgenase (LDH), and FLIPI score (Table 12). Overall survival did not differ between the two treatment groups.
The rate of overall response did not differ between the treatment groups (93% for B-R vs. 91% for R-CHOP); however the rate of complete response was significantly increased in patients in the B-R group (104 [40%] vs. 76 [30%]; p=0.021).
Table 12: Exploratory subgroup analysis to assess the PFS benefit of B-R vs. R-CHOP:
| HR (95% Cl) | p value | |
|---|---|---|
| Age (years) | ||
| ≤ 60 (n=199) | 0.52 (0.33-0.79) | 0.002 |
| > 60 (n=315) | 0.62 (0.45-0.84) | 0.002 |
| LDH concentration | ||
| Normal (n=319) | 0.48 (0.34-0.67) | <0.0001 |
| Elevated (n=184) | 0.74 (0.50-1.08) | 0.118 |
| FLIPI subgroup | ||
| Favourable (0-2 risk factors; n=143) | 0.56 (0.31-0.98) | 0.043 |
| Unfavourable (3-5 risk factors; n=127) | 0.63 (0.38-1.04) | 0.068 |
PFS=progression-free survival; LDH=lactate dehydrogenase; FLIPI=Follicular Lymphoma International Prognostic Index; HR=hazard ratio.
Relapsed / Refractory NHL
The efficacy of RIBOMUSTIN was evaluated in a single arm study (SDX-105-03) of 100 patients with indolent B-cell NHL that had progressed during or within six months of treatment with rituximab or a rituximab-containing regimen. Patients were included if they relapsed within 6 months of either the first dose (monotherapy) or last dose (maintenance regimen or combination therapy) of rituximab. All patients received RIBOMUSTIN intravenously at a dose of 120 mg/m², on Days 1 and 2 of a 21-day treatment cycle. Patients were treated for up to 8 cycles.
The median age was 60 years, 65% were male, and 95% had a baseline WHO performance status of 0 or 1. Major tumour subtypes were follicular lymphoma (62%), diffuse small lymphocytic lymphoma (21%), and marginal zone lymphoma (16%). Ninety-nine percent of patients had received previous chemotherapy, 91% of patients had received previous alkylator therapy, and 97% of patients had relapsed within 6 months of either the first dose (monotherapy) or last dose (maintenance regimen or combination therapy) of rituximab. Efficacy was based on the assessments by a blinded independent review committee (IRC) and included overall response rate (complete response + complete response unconfirmed + partial response) and duration of response (DR) as summarized in Table 13.
Table 13. Efficacy data for progressing NHL* reported in study SDX-105-03:
| RIBOMUSTIN (N=100) | |
|---|---|
| Response Rate (%) | |
| Overall response rate (CR+CRu+PR) | 74 |
| (95% CI) | (64.3, 82.3) |
| Complete response (CRu) | 13 |
| Partial response unconfirmed (CRu) | 4 |
| Partial response (PR) | 57 |
| Duration of Response (DR) | |
| Median, month (95% CI) | 9.2 months (7.1, 10.8) |
CI = confidence interval
* IRC assessment was based on modified International Working Group response criteria (IWG-RC). Modifications to IWG-RC specified that a persistently positive bone marrow in patients who met all other criteria for CR would be scored as PR. Bone marrow sample lengths were not required to be ≥20 mm.
Progression-free survival (PFS), a secondary endpoint in this study, was comparable across all patient groups defined by baseline characteristics (Table 14). The median PFS was 72 weeks in patients without previous alkylator therapy, and 51 weeks in patients who were sensitive to the previous alkylator therapy or chemotherapy. In the patients who had received previous radioimmunotherapy, the PFS was 53 weeks. Disease characteristics at baseline (FLIPI risk category, number of lymph nodal sites, or bulky disease) did not markedly affect duration of PFS.
Table 14. Progression-Free Survival by Baseline Characteristics:
| Response/Baseline Characteristics | RIBOMUSTIN (N=100) Median, weeks (95% CI) |
|---|---|
| Best response | |
| Complete response (n=14) | 51.1 (46.3, 56.7) |
| Unconfirmed complete response (n=3) | 64.9 (35.0, NA) |
| Partial response (n=58) | 42.3 (35.9, 53.3) |
| Previous alkylator therapy | |
| With previous alkylator therapy (n=91) | 36.3 (33.4, 51.1) |
| Without previous alkylator therapy (n=9) | 71.6 (36.6, 71.6) |
| Sensitivity to last alkylator therapy | |
| Sensitive (n=51) | 51.1 (36.3, 56.7) |
| Refractory (n=36) | 32.7 (19.1, 52.3) |
| Unknown (n=10) | 30.0 (20.6, 35.0) |
| Sensitivity to last chemotherapy therapy | |
| Sensitive (n=51) | 51.1 (39.0, 56.7) |
| Refractory (n=36) | 32.7 (19.1, 52.3) |
| Unknown (n=12) | 30.1 (20.6, 42.4) |
| Number of previous chemotherapy courses | |
| ≤3 courses (n=92) | 42.4 (35.0, 52.3) |
| >3 courses (n=8) | 30.1 (24.3, 35.9) |
| Previous radioimmunotherapy | |
| With previous radioimmunotherapy (n=24) | 53.3 (34.7, 71.6) |
| Without previous radioimmunotherapy (n=76) | 39.0 (34.0, 51.1) |
| Follicular Lymphoma International Prognostic Index (risk category) | |
| Low rsik (n=18) | 40.3 (32.7, 51.9) |
| Intermediate risk (n=26) | 39.0 (30.1, NA) |
| High risk (n=18) | 35.6 (27.4, 54.1) |
| Number of lymph nodal sites | |
| ≤4 involved lymph nodes (n=52) | 40.3 (32.0, 51.9) |
| >4 involved lymph nodes (n=48) | 46.3 (34.0, 54.1) |
| Bulky disease at baseline | |
| Lymph nodes <10 cm (n=89) | 42.3 (35.0, 51.9) |
| Lymph nodes ≥10 cm (n=8) | 40.3 (6.1, NA) |
CI = confidence interval; NA = not applicable
5.2. Pharmacokinetic properties
Distribution
Following 30 min I.V. infusion the central volume of distribution was 19.3 L. Under steadystate conditions following I.V. bolus injection the volume of distribution was 15.8-20.5 L. More than 95% of the substance is bound to plasma proteins (primarily albumin).
Metabolism
A major route of clearance of bendamustine is the hydrolysis to monohydroxy- and dihydroxybendamustine. Formation of N-desmethyl-bendamustine and gamma-hydroxy-bendamustine by hepatic metabolism involves cytochrome P450 (CYP) 1A2 isoenzyme. Another major route of bendamustine metabolism involves conjugation with glutathione. In-vitro bendamustine does not inhibit CYP 1A2, CYP 2C9/10, CYP 2D6, CYP 2E1 and CYP 3A4.
Elimination
the elimination half-life t1/2ß after 30 min I.V. infusion of 120 mg/m² to 12 subjects was 28.2 minutes. The mean total clearance after 30 min I.V. infusion of 120 mg/m² body surface area to 12 subjects was 639.4 mL/minute. About 20% of the administered dose was recovered in urine within 24 hours. Amounts excreted in urine were in the order monohydroxybendamustine > bendamustine > dihydroxy-bendamustine > oxidised metabolite > N-desmethyl bendamustine. In the bile, primarily polar metabolites are eliminated.
Special populations
Renal Impairment
In patients with creatinine clearance >10 mL/min including dialysis dependent patients, no significant difference to patients with normal liver and kidney function was observed with respect to Cmax, tmax, AUC, t1/2ß, volume of distribution and clearance.
Hepatic Impairment
In patients with 30-70% tumour infestation of the liver and mild hepatic impairment (serum bilirubin <1.2 mg/dL) the pharmacokinetic behaviour was not changed. There was no significant difference to patients with normal liver and kidney function with respect to Cmax, tmax, AUC, t1/2ß, volume of distribution and clearance. AUC and total body clearance of bendamustine correlate inversely with serum bilirubin.
Elderly subjects
Subjects up to 84 years of age were included in pharmacokinetic studies. Higher age does not influence the pharmacokinetics of bendamustine.
5.3. Preclinical safety data
Genotoxicity
Animal studies showed that bendamustine is embryotoxic and teratogenic. Bendamustine induces aberrations of the chromosomes and is mutagenic in-vivo as well as in-vitro. Adverse reactions not observed in clinical studies, but seen in animals at exposure levels similar to clinical exposure levels and with possible relevance to clinical use were as follows: histological investigations in dogs showed macroscopic visible hyperaemia of the mucosa and haemorrhagia in the gastrointestinal tract. Microscopic investigations showed extensive changes of the lymphatic issue indicating an immunosuppression and tubular changes of kidneys and testis, as well as atrophic, necrotic changes of the prostate epithelium.
Carcinogenicity
In long-term studies in female mice bendamustine is carcinogenic.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.