RIBOMUSTIN Powder for solution for infusion Ref.[50298] Active ingredients: Bendamustine
Source: Marketing Authorisation Holder Revision Year: 2022 Publisher: Janssen-Cilag (New Zealand) Ltd, Auckland, NEW ZEALAND, Telephone: 0800 800 806, Fax: (09) 588 1398, Email: medinfo@janau.jnj.com
4.3. Contraindications
RIBOMUSTIN is contraindicated in patients with:
- Hypersensitivity to the active substance or to any of the excipients.
- Severe hepatic impairment (serum bilirubin >3.0 mg/dL)
- Jaundice
- Severe bone marrow suppression and severe blood count alteration (leukocyte and/or platelet values dropped to <3 x 109/L or <75 x 109/L, respectively)
- Major surgery less than 30 days before start of treatment
- Infections, especially involving leukocytopaenia
- Yellow fever vaccination
- RIBOMUSTIN is also contraindicated during breast-feeding
4.4. Special warnings and precautions for use
Myelosuppression
Patients treated with RIBOMUSTIN may experience bone marrow failure. In the event of treatment-related myelosuppression, leukocytes, platelets, haemoglobin, and neutrophils must be monitored at least weekly. Treatment-related myelosuppression may require dose adjustment and/or dose delays.
Treatment with bendamustine hydrochloride may cause prolonged lymphocytopenia (<0.6 x 109/L) and low CD4-positive T-cell (T-helper cell) counts (<0.2 x 109/L) for at least 7-9 months after the completion of treatment. Lymphocytopenia and CD4-positive T-cell depletion are more pronounced when bendamustine is combined with rituximab. Patients with lymphopenia and low CD4-positive T-cell count following treatment with bendamustine hydrochloride are more susceptible to (opportunistic) infections.
Prior to the initiation of the next cycle of therapy, the following parameters are recommended: Leukocyte and/or platelet values >4 x 109/L or >100 x 109/L, respectively. RIBOMUSTIN should not be used during severe bone marrow suppression and severe blood count alterations (see section 4.2).
Infections
Serious and fatal infections, including fatal sepsis, have occurred with bendamustine treatment. These infections included bacterial (pneumonia) and opportunistic infections such as Pneumocystis Jirovecii Pneumonia (PJP), Varicella Zoster Virus (VZV), Cytomegalovirus (CMV), and progressive multifocal leukoencephalopathy (John Cunningham virus). Cases of progressive multifocal leukoencephalopathy (PML) including fatal ones have been reported following the use of bendamustine mainly in combination with rituximab or Obinutuzumab.
Patients with lymphopenia and low CD4-positive T-cell count following treatment with bendamustine hydrochloride are more susceptible to (opportunistic) infections. In case of low CD4-positive T-cell counts (<0.2 x 109/L) Pneumocystis jirovecii pneumonia (PJP) prophylaxis should be considered.
Consider PML in the differential diagnosis in patients with new or worsening neurological, cognitive or behavioural signs or symptoms. If PML is suspected then appropriate diagnostic evaluations should be undertaken and treatment suspended until PML is excluded.
All patients should be monitored for respiratory signs and symptoms throughout treatment. Discontinuation of bendamustine hydrochloride should be considered if there are signs of (opportunistic) infections.
Hepatitis B reactivation
Reactivation of hepatitis B in patients who are chronic carriers of this virus has occurred after these patients received bendamustine hydrochloride. Some cases resulted in acute hepatic failure or a fatal outcome. Patients should be tested for HBV infection before initiating treatment with bendamustine hydrochloride. For patients with hepatitis B serology indicative of prior infection, a liver disease expert should be consulted before the start of treatment and the patient should be monitored and managed following local medical standards to prevent hepatitis B reactivation. Monitoring should continue for several months following termination of therapy (see section 4.8).
Skin Reactions
A number of skin reactions have been reported. These events have included rash, severe cutaneous reactions and bullous exanthema. Cases of Stevens – Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), some fatal, have also been reported. Some events of SJS and TEN occurred when bendamustine hydrochloride was administered concomitantly with allopurinol or when bendamustine hydrochloride was given in combination with other anticancer agents. Cases of drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported with the use of bendamustine hydrochloride in combination with rituximab. Patients should be advised of the signs and symptoms of these reactions by their prescribers and should be told to seek medical attention immediately if they develop these symptoms. Where skin reactions occur, they may be progressive and increase in severity with further treatment. If skin reactions are progressive, RIBOMUSTIN should be withheld or discontinued. For severe skin reactions where a relationship to bendamustine hydrochloride is suspected, treatment should be discontinued.
Patients with Cardiac Disorders
During treatment with RIBOMUSTIN the concentration of potassium in the blood must be closely monitored and potassium supplement must be given when K+ <3.5 mEq/l, and ECG measurement must be performed. Fatal cases of myocardial infarction and cardiac failure have been reported with bendamustine treatment. Patients with concurrent or history of cardiac disease should be observed closely.
Nausea, vomiting
An antiemetic may be given for the symptomatic treatment of nausea and vomiting.
Tumour Lysis Syndrome
Tumour lysis syndrome associated with RIBOMUSTIN treatment has been reported in patients in clinical trials. The onset tends to be within 48 hours of the first dose of RIBOMUSTIN and, without intervention, may lead to acute renal failure and death. Preventive measures include adequate volume status, close monitoring of blood chemistry, particularly potassium and uric acid levels. The use of allopurinol during the first one to two weeks of RIBOMUSTIN therapy can be considered but not necessarily as standard. However, there have been a few cases of Stevens-Johnson Syndrome and Toxic Necrolysis reported when bendamustine and allopurinol are concomitantly administered.
Anaphylaxis
Infusion reactions to bendamustine hydrochloride have occurred in clinical trials. Symptoms are generally mild and include fever, chills, pruritus and rash. In rare instances severe anaphylactic and anaphylactoid reactions have occurred. Patients must be asked about symptoms suggestive of infusion reactions after their first cycle of therapy. Measures to prevent severe reactions, including antihistamines, antipyretics and corticosteroids must be considered in subsequent cycles in patients who have previously experienced infusion reactions. Patients who experienced Grade 3 or worse allergic-type reactions were typically not rechallenged.
Extravasation
An extravasal injection should be stopped immediately. The needle should be removed after a short aspiration. Thereafter the affected area of tissue should be cooled. The arm should be elevated. Additional treatments like the use of corticosteroids are not of clear benefit.
Non-melanoma skin cancer
In clinical studies, an increased risk for non-melanoma skin cancers (basal cell carcinoma and squamous cell carcinoma) has been observed in patients treated with bendamustine containing therapies. Periodic skin examination is recommended for all patients, particularly those with risk factors for skin cancer.
Other Malignancies
The risk of myelodysplastic syndrome and acute myeloid leukaemias is increased in patients treated with alkylating agents (including bendamustine). The secondary malignancy may develop several years after chemotherapy has been discontinued.
Paediatric Use
There is no experience in children and adolescents with RIBOMUSTIN.
4.5. Interaction with other medicinal products and other forms of interaction
No in-vivo interaction studies have been performed.
When RIBOMUSTIN is combined with myelosuppresive agents, the effect of RIBOMUSTIN and/or the co-administered medicinal products on the bone marrow may be potentiated. Any treatment reducing the patient's performance status or impairing bone marrow function can increase the toxicity of RIBOMUSTIN.
Combination of RIBOMUSTIN with cyclosporine or tacrolimus may result in excessive immunosuppression with risk of lymphoroliferation.
Cytostatics can reduce antibody formation following live-virus vaccination and increase the risk of infection which may lead to fatal outcome. This risk is increased in subjects who are already immunosuppressed by their underlying disease.
Bendamustine metabolism involves cytochrome P450 (CYP) 1A2 isoenzyme (see section 5.2). Therefore, potential for interaction with CYP1A2 inhibitors such as fluvoxamine, ciprofloxacin, acyclovir, cimetidine exists.
4.6. Fertility, pregnancy and lactation
Use in pregnancy
Category C.
There are insufficient data from the use of RIBOMUSTIN in pregnant women. In nonclinical studies bendamustine was embryo-/feto lethal, teratogenic and genotoxic.
During pregnancy RIBOMUSTIN should not be used unless clearly necessary. The mother should be informed about the risk to the foetus. If treatment with RIBOMUSTIN is absolutely necessary during pregnancy or if pregnancy occurs during treatment, the patient should be informed about the risks for the unborn child and be monitored carefully. The possibility of genetic counselling should be considered.
Women of childbearing potential must use effective methods of contraception both before and during RIBOMUSTIN therapy.
Men being treated with RIBOMUSTIN are advised not to father a child during and for up to 6 months following cessation of treatment. Advice on conservation of sperm should be sought prior to treatment because of the possibility of irreversible infertility due to therapy with RIBOMUSTIN.
Use in lactation
It is not known whether bendamustine passes into the breast milk, therefore, bendamustine is contraindicated during breast-feeding (see section 4.3). Breast-feeding must be discontinued during treatment with bendamustine.
4.7. Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. However, somnolence has been reported during treatment with RIBOMUSTIN (see section 4.8). Patients should be instructed that if they experience these symptoms they should avoid potentially hazardous tasks such as driving and using machines.
4.8. Undesirable effects
Clinical Trials Data
Chronic Lymphocytic Lymphoma
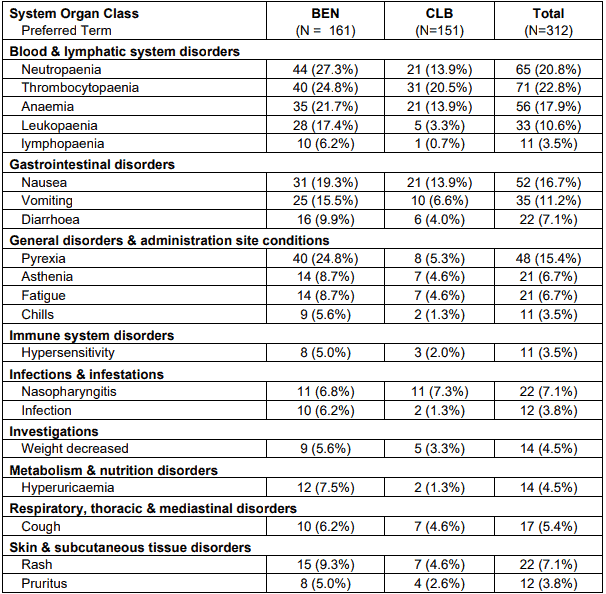
The following tables describe the safety results reported in study 02CLLIII of 161 previouslyuntreated patients with Binet Stage B or C CLL requiring treatment.
Table 1. Adverse events occurring in at least 5% of patients in either treatment group by system organ class and preferred term - safety population:
Note: Patients are counted only once in each preferred term category and only once in each system organ class. Within each system organ class preferred terms were sorted descending according the frequency in the BEN group.
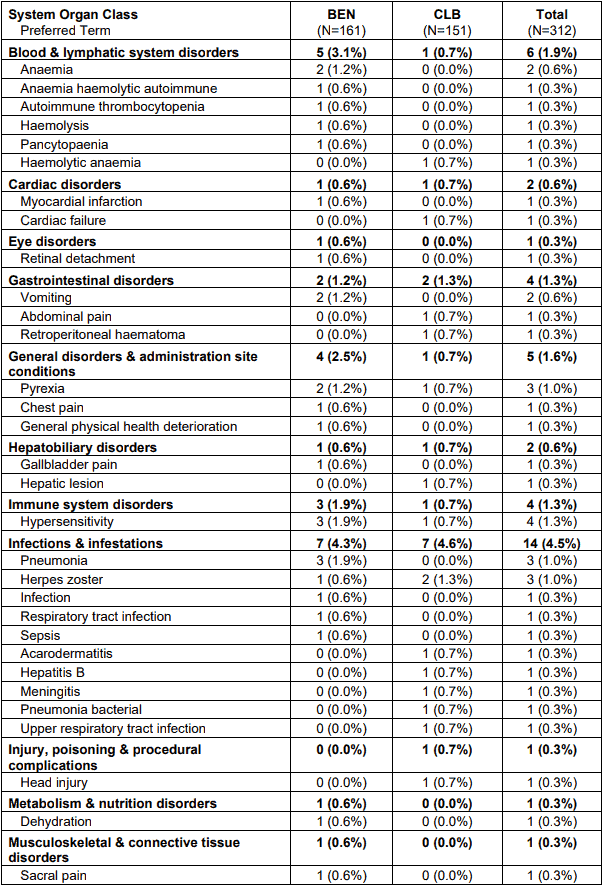
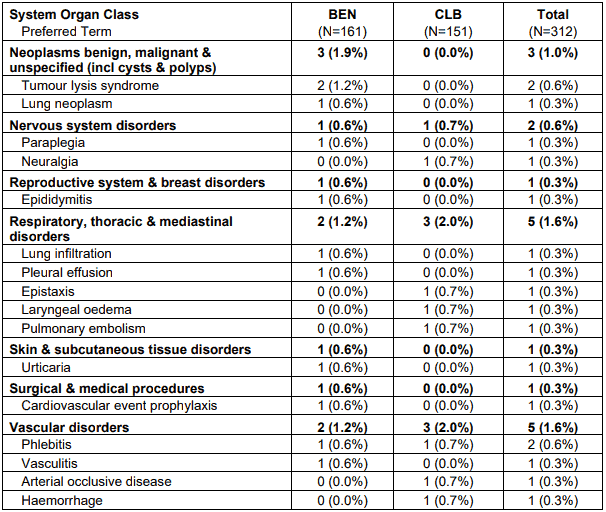
A total of 50 patients had 60 serious adverse events. Most frequently occurring serious adverse events in the bendamustine group were hypersensitivity and pneumonia (each with 3 patients) and anemia, vomiting, pyrexia and tumour lysis syndrome (each with 2 patients). Most frequent documented serious adverse event in the chlorambucil group was herpes zoster (with 2 patients). All other events were documented only once by patient.
Table 2. Serious adverse events by system organ class and preferred term - safety population:
Note: Patients are counted only once in each preferred term category and only once in each system organ class. Within each system organ class preferred terms were sorted descending according the frequency in the BEN group.
Number of adverse events possible, probable or definite related to the study medication (including missing relationship) was higher in the bendamustine arm than in the chlorambucil arm. Especially blood and lymphatic system disorders, gastrointestinal disorders and pyrexia occurred more frequently under bendamustine than under chlorambucil.
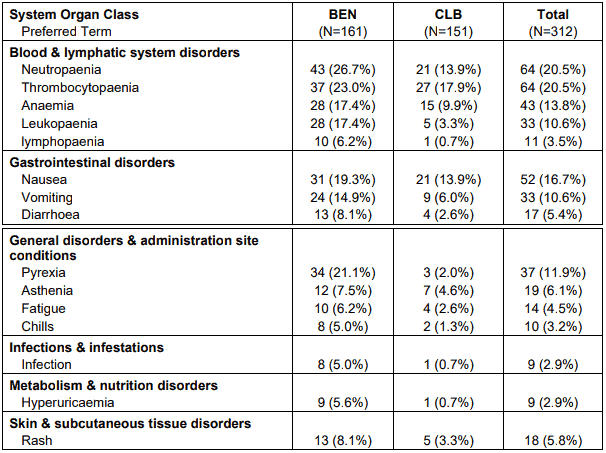
Table 3. Drug related adverse events by CTC category in at least 5% of patients in either treatment group by system organ class and preferred term – safety population:
Note: Patients are counted only once in each preferred term category and only once in each system organ class.
The most frequent adverse reactions leading to study withdrawal for patients receiving RIBOMUSTIN were hypersensitivity (2%) and pyrexia (1%).
Results from the NHL1-2003 Clinical Trial in Patients with Previously Untreated Advanced Indolent Non-Hodgkin's Lymphoma and Mantle Cell Lymphoma:
Table 4 and Table 5 describe safety data from the NHL1-2003 study with previously untreated advanced indolent NHL who received RIBOMUSTIN IV (90 mg/m²) in combination with rituximab (375 mg/m²).
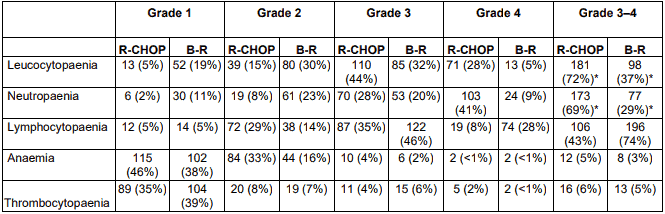
Table 4. Haematological toxic events in patients receiving at least one dose of study treatment:
BR=bendamustine plus rituximab;
R-CHOP=CHOP plus rituximab;
* p <0.0001 between groups.
Table 5. All grades of non-haematological toxic events in patients receiving at least one dose of study treatment:
| B-R (n=261) | R-CHOP (n=253) | p value | |
|---|---|---|---|
| Alopecia | 0 | 245 (100%)* | <0.0001 |
| Parethesia | 18 (7%) | 73 (29%) | <0.0001 |
| Stomatitis | 16 (6%) | 47 (19%) | <0.0001 |
| Skin (erythema) | 42 (16%) | 23 (9%) | 0.024 |
| Skin (allergic reaction) | 40 (15%) | 15 (6%) | 0.0006 |
| Infectious episodes | 96 (37%) | 127 (50%) | 0.0025 |
| Sepsis | 1 (<1%) | 8 (3%) | 0.019 |
B-R=bendamustine plus rituximab;
R-CHOP=CHOP plus rituximab.
* Includes only patients who received three or more cycles
Relapsed / Refractory Non-Hodgkin's Lymphoma
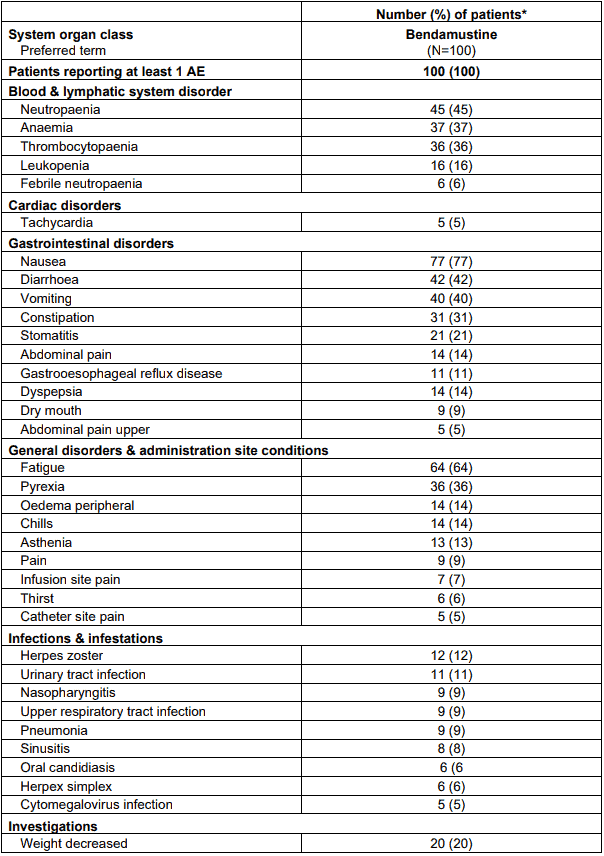
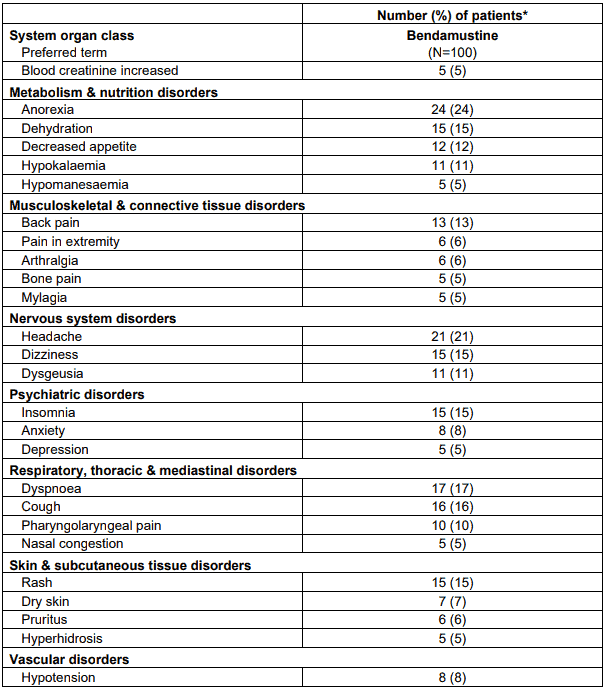
Table 6 lists adverse events occurring in at least 5% of patients in study SDX-105-03.
Table 6. Adverse events occurring in at least 5% of patients by preferred term:
* Patients are counted only once for each arm
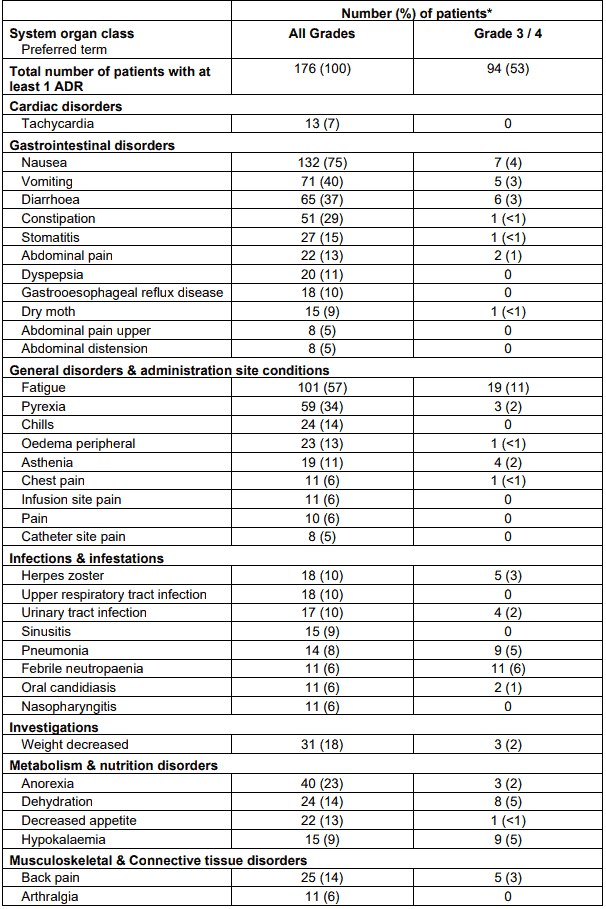
The data described below reflect exposure to RIBOMUSTIN in 176 patients with indolent B-cell NHL treated in two single-arm studies (SDX-105-03 and SDX-105-01).
The adverse reactions occurring in at least 5% of the NHL patients, regardless of severity, are shown in Table 7.
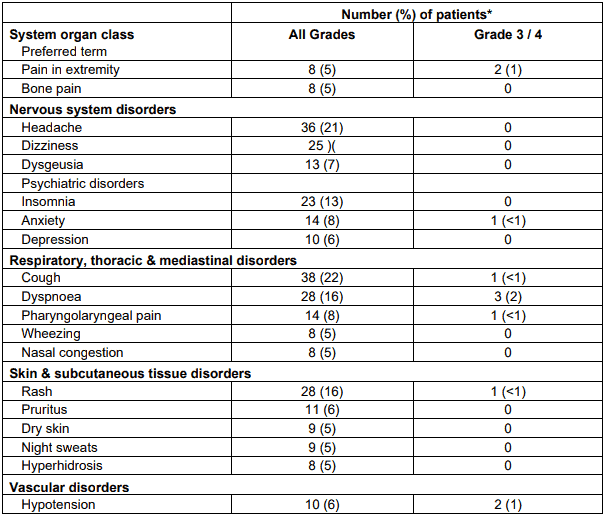
Table 7. Non-haematological ADRs occurring in at least 5% of NHL patients treated with RIBOMUSTIN by system organ class and preferred term:
* Patients may have reported more than 1 adverse reaction.
NOTE: Patients counted only once in each preferred term category and once in each system organ class category.
Haematologic toxicities, based on laboratory values and CTC grade, in NHL patients treated in both single arm studies combined are described in Table 11. Clinically important chemistry laboratory values that were new or worsened from baseline and occurred in >1% of patients at Grade 3 or 4, in NHL patients treated in both single arm studies combined were hyperglycemia (3%), elevated creatinine (2%), hyponatremia (2%), and hypocalcemia (2%).
Table 8. Incidence of haematology laboratory abnormalities in patients who received RIBOMUSTIN in the NHL studies:
| Percent of patients | ||
|---|---|---|
| Haematology variable | All Grades | Grade 3/4 |
| Lymphocytes decreased | 99 | 94 |
| Leukocytes decreased | 94 | 56 |
| Haemoglobin decreased | 88 | 11 |
| eutrophils decreased | 86 | 60 |
| Platelets decreased | 86 | 25 |
In both studies, serious adverse reactions, regardless of causality, were reported in 37% of patients receiving RIBOMUSTIN. The most common serious adverse reactions occurring in 5% of patients were febrile neutropaenia and pneumonia. Other important serious adverse reactions reported in clinical trials and/or postmarketing experience were acute renal failure, cardiac failure, hypersensitivity, skin reactions, pulmonary fibrosis, pneumonitis, pulmonary alveolar haemorrhage and myelodysplastic syndrome.
Serious drug-related adverse reactions reported in clinical trials included myelosuppression, infection, pneumonia, tumour lysis syndrome and infusion reactions (see section 4.4).
Adverse reactions occurring less frequently but possibly related to RIBOMUSTIN treatment were haemolysis, dysgeusia/taste disorder, a typical pneumonia, sepsis, herpes zoster, erythema, dermatitis, and skin necrosis.
Post Marketing Experience
The following adverse reactions have been identified during post-approval use of
RIBOMUSTIN (Table 9).
Very common ≥1/10
Common ≥1/100 and <1/10
Uncommon ≥ 1/ 1000 and <1/100
Rare ≥1/10000 and <1/1000
Very rare <1/10000, including isolated reports.
Table 9. Adverse Reactions Identified During Postmarketing Experience with Ribosmustin:
| Skin and subcutaneous tissue disorders | |
| Not known | Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) |
| Common | Urticaria |
| Respiratory, thoracic and mediastinal disorders | |
| Not known | Pneumonitis, pulmonary alveolar haemorrhage |
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: anaphylaxis; and injection or infusion site reactions including phlebitis, pruritus, irritation, pain, and swelling. Increases in alanine aminotransferase; aspartate aminotransferase; blood bilirubin and blood urea levels have been reported. Somnolence, atrial fibrillations and palpitations have also been reported.
Skin reactions including Stevens-Johnson Syndrome (SJS), Toxic Epidermal Necrolysis (TEN), some fatal, have been reported with the use of bendamustine hydrochloride (see PRECAUTIONS – Skin Reactions and Tumour Lysis Syndrome, see section 4.4). In addition, a few cases of hepatitis B reactivation resulting in hepatic failure have been reported in patients treated with bendamustine. Pancytopenia, headache, dizziness, opportunistic infection (e.g. herpes zoster, cytomegalovirus, pneumocystis jirovecii pneumonia), bone marrow failure, hepatic failure, renal failure, nephrogenic diabetes insipidus, drug reaction with eosinophilia and systemic symptoms (combination therapy with rituximab) have also been reported in patients treated with bendamustine.
The CD4/CD8 ratio may be reduced. A reduction of the lymphocyte count was seen. In immunosuppressed patients, the risk of infection (e.g., with herpes zoster) may be increased.
There have been isolated reports of necrosis after accidental extra-vascular administration, tumour lysis syndrome, and anaphylaxis.
The risk of myelodysplastic syndrome and acute myeloid leukaemias is increased in patients treated with alkylating agents (including bendamustine). The secondary malignancy may develop several years after chemotherapy has been discontinued.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicine is important. It allows continued monitoring of the benefit/risk balance of the medicine. Healthcare professionals are asked to report any suspected adverse reactions https//nzphvc.otago.ac.nz/reporting/
6.2. Incompatibilities
In the absence of data bendamustine should not be mixed with other medicinal products, except those mentioned in section 6.6.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.