RISPERDAL CONSTA Powder and solvent for suspension for injection Ref.[7351] Active ingredients: Risperidone
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2018 Publisher: Janssen-Cilag Ltd, 50-100 Holmers Farm Way, High Wycombe, Bucks, HP12 4EG, UK
Pharmacodynamic properties
Pharmacotherapeutic group: Other antipsychotics
ATC code: N05AX08
Mechanism of action
Risperidone is a selective monoaminergic antagonist with unique properties. It has a high affinity for serotoninergic 5-HT2 and dopaminergic D2 receptors. Risperidone binds also to alpha-1-adrenergic receptors, and, with lower affinity, to H1-histaminergic and alpha-2-adrenergic receptors. Risperidone has no affinity for cholinergic receptors. Although risperidone is a potent D2 antagonist, that is considered to improve the positive symptoms of schizophrenia, it causes less depression of motor activity and induction of catalepsy than classical antipsychotics. Balanced central serotonin and dopamine antagonism may reduce extrapyramidal side effect liability and extend the therapeutic activity to the negative and affective symptoms of schizophrenia.
Clinical efficacy
The effectiveness of RISPERDAL CONSTA (25 mg and 50 mg) in the management of the manifestations of psychotic disorders (schizophrenia/schizoaffective disorder) was established in one 12-week, placebo-controlled trial in adult psychotic inpatients and outpatients who met the DSM-IV criteria for schizophrenia.
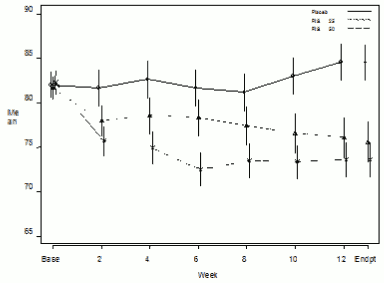
In a 12-week comparative trial in stable patients with schizophrenia, RISPERDAL CONSTA was shown to be as effective as the oral tablet formulation. The long-term (50 weeks) safety and efficacy of RISPERDAL CONSTA was also evaluated in an open-label trial of stable psychotic inpatients and outpatients who met the DSM-IV criteria for schizophrenia or schizoaffective disorder. Over time efficacy was maintained with RISPERDAL CONSTA (Figure 1).
Figure 1. Mean in total PANSS score over time (LOCF) in patients with schizophrenia:
Pharmacokinetic properties
Absorption
The absorption of risperidone from RISPERDAL CONSTA is complete.
After a single intramuscular injection with RISPERDAL CONSTA, the release profile consists of a small initial release of risperidone (<1% of the dose), followed by a lag time of 3 weeks. The main release of risperidone starts from week 3 onwards, is maintained from 4 to 6 weeks, and subsides by week 7. Oral antipsychotic supplementation should therefore be given during the first 3 weeks of RISPERDAL CONSTA treatment (see section 4.2).
The combination of the release profile and the dosage regimen (intramuscular injection every two weeks) results in sustained therapeutic plasma concentrations. Therapeutic plasma concentrations remain until 4 to 6 weeks after the last RISPERDAL CONSTA injection.
After repeated intramuscular injections with 25 or 50 mg RISPERDAL CONSTA every two weeks, median trough and peak plasma concentrations of the active antipsychotic fraction fluctuated between 9.9-19.2 ng/ml and 17.9-45.5 ng/ml respectively. No accumulation of risperidone was observed during long term use (12 months) in patients who were injected with 25–50 mg every two weeks.
The above studies were conducted with gluteal intramuscular injection. Deltoid and gluteal intramuscular injections at the same doses are bioequivalent and, therefore, interchangeable.
Distribution
Risperidone is rapidly distributed. The volume of distribution is 1-2 l/kg. In plasma, risperidone is bound to albumin and alpha1-acid glycoprotein. The plasma protein binding of risperidone is 90%; that of the active metabolite 9-hydroxy-risperidone is 77%.
Biotransformation and elimination
Risperidone is metabolised by CYP2D6 to 9-hydroxy-risperidone, which has a similar pharmacological activity as risperidone. Risperidone plus 9-hydroxy-risperidone form the active antipsychotic fraction. CYP2D6 is subject to genetic polymorphism. Extensive CYP2D6 metabolisers convert risperidone rapidly into 9-hydroxy-risperidone, whereas poor CYP2D6 metabolisers convert it much more slowly. Although extensive metabolisers have lower risperidone and higher 9-hydroxy-risperidone concentrations than poor metabolisers, the pharmacokinetics of risperidone and 9-hydroxy-risperidone combined (i.e. the active antipsychotic fraction), after single and multiple doses, are similar in extensive and poor metabolisers of CYP2D6.
Another metabolic pathway of risperidone is N-dealkylation. In vitro studies in human liver microsomes showed that risperidone at clinically relevant concentration does not substantially inhibit the metabolism of medicines metabolised by cytochrome P450 isozymes, including CYP1A2, CYP2A6, CYP2C8/9/10, CYP2D6, CYP2E1, CYP3A4, and CYP3A5. One week after oral risperidone administration, 70% of the dose is excreted in the urine and 14% in the faeces. In urine, risperidone plus 9-hydroxy-risperidone represent 35-45% of the orally administered dose. The remainder is inactive metabolites. The elimination phase is complete approximately 7 to 8 weeks after the last RISPERDAL CONSTA injection.
Linearity
The pharmacokinetics of risperidone are linear in the dose range of 25-50 mg injected every 2 weeks.
Elderly, hepatic and renal impairment
A single-dose PK-study with oral risperidone showed on average a 43% higher active antipsychotic fraction plasma concentrations, a 38% longer half-life and a reduced clearance of the active antipsychotic fraction by 30% in the elderly.
In adults with moderate renal disease the clearance of the active moiety was ~48% of the clearance in young healthy adults (age range 25-35 years). In adults with severe renal disease the clearance of the active moiety was ~31% of the clearance in young healthy adults. The half-life of the active moiety was 16.7 h in young adults, 24.9 h in adults with moderate renal disease (or ~1.5 times as long as in young adults), and 28.8 h in those with severe renal disease (or ~1.7 times as long as in young adults). Risperidone plasma concentrations were normal in patients with liver insufficiency, but the mean free fraction of risperidone in plasma was increased by 37.1%.
The oral clearance and the elimination half-life of risperidone and of the active moiety in adults with moderate and severe liver impairment were not significantly different from those parameters in young healthy adults.
Pharmacokinetic/pharmacodynamic relationship
There was no relationship between the plasma concentrations of the active antipsychotic fraction and the change in total PANSS (Positive And Negative Syndrome Scale) and total ESRS (Extrapyramidal Symptom Rating Scale) scores across the assessment visits in any of the phase III trials where efficacy and safety was examined.
Gender, race and smoking habits
A population pharmacokinetic analysis revealed no apparent effect of gender, race or smoking habits on the pharmacokinetics of risperidone or the active antipsychotic fraction.
Preclinical safety data
Similar to the (sub)chronic toxicity studies with oral risperidone in rats and dogs, the major effects of treatment with RISPERDAL CONSTA (up to 12 months of intramuscular administration) were prolactin-mediated mammary gland stimulation, male and female genital tract changes, and central nervous system (CNS) effects, related to the pharmacodynamic activity of risperidone. In a toxicity study in juvenile rats treated with oral risperidone, increased pup mortality and a delay in physical development was observed. In a 40-week study with juvenile dogs treated with oral risperidone, sexual maturation was delayed. Based on AUC, long bone growth was not affected in dogs at 3.6-times the maximum human oral exposure in adolescents (1.5 mg/day); while effects on long bones and sexual maturation were observed at 15 times the maximum human oral exposure in adolescents.
Risperidone was not teratogenic in rat and rabbit. In rat reproduction studies with risperidone, adverse effects were seen on mating behaviour of the parents, and on birth weight and survival of the offspring. In rats, intrauterine exposure to risperidone was associated with cognitive deficits in adulthood. Other dopamine antagonists, when administered to pregnant animals, have caused negative effects on learning and motor development in the offspring.
RISPERDAL CONSTA administration to male and female rats for 12 and 24 months produced osteodystrophy at a dose of 40 mg/kg/2 weeks. The effect dose for osteodystrophy in rats was on a mg/m² basis 8 times the maximum recommended human dose and is associated with a plasma exposure 2 times the maximum anticipated exposure in humans at the maximum recommended dose. No osteodystrophy was observed in dogs treated for 12 months with RISPERDAL CONSTA up to 20 mg/kg/2 weeks. This dose yielded plasma exposures up to 14 times the maximum recommended human dose.
There was no evidence of genotoxic potential.
As expected for a potent dopamine D2 antagonist, in oral carcinogenicity studies of risperidone in rats and mice, increases in pituitary gland adenomas (mouse), endocrine pancreas adenomas (rat), and mammary gland adenomas (both species) were seen.
In an intramuscular carcinogenicity study with RISPERDAL CONSTA in Wistar (Hannover) rats (doses of 5 and 40 mg/kg/2 weeks), increased incidences of endocrine pancreas, pituitary gland, and adrenal medullary tumours were observed at 40 mg/kg, while mammary gland tumours were present at 5 and 40 mg/kg. These tumours observed upon oral and intramuscular dosing can be related to prolonged dopamine D2 antagonism and hyperprolactinaemia. Tissue culture studies suggest that cell growth in human breast tumours may be stimulated by prolactin. Hypercalcemia, postulated to contribute to an increased incidence of adrenal medullary tumours in RISPERDAL CONSTA-treated rats, was observed in both dose groups. There is no evidence to suggest that hypercalcemia might cause phaeochromocytomas in humans.
Renal tubular adenomas occurred in male rats treated with RISPERDAL CONSTA at 40 mg/kg/2 weeks. No renal tumours occurred in the low dose, the NaCl 0.9%, or the microspheres vehicle control group. The mechanism underlying the renal tumours in RISPERDAL CONSTA-treated male Wistar (Hannover) rats is unknown. A treatment-related increase in renal tumour incidence did not occur in the oral carcinogenicity studies with Wistar (Wiga) rats or in Swiss mice administered oral risperidone. Studies conducted to explore the substrain differences in the tumour organ profile suggest that the Wistar (Hannover) substrain employed in the carcinogenicity study differs substantially from the Wistar (Wiga) substrain employed in the oral carcinogenicity study with respect to spontaneous age-related non-neoplastic renal changes, serum prolactin increases, and renal changes in response to risperidone. There are no data suggesting kidney-related changes in dogs treated chronically with RISPERDAL CONSTA.
The relevance of the osteodystrophy, the prolactin-mediated tumours and of the presumed rat substrain-specific renal tumours in terms of human risk is unknown.
Local irritation at the injection site in dogs and rats was observed after administration of high doses of RISPERDAL CONSTA. In a 24-month intramuscular carcinogenicity study in rats, no increased incidence of injection site tumours was seen in either the vehicle or active groups.
In vitro and in vivo, animal models show that at high doses risperidone may cause QT interval prolongation, which has been associated with a theoretically increased risk of Torsade de Pointes in patients.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.