RISPERDAL CONSTA Powder and solvent for suspension for injection Ref.[7351] Active ingredients: Risperidone
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2018 Publisher: Janssen-Cilag Ltd, 50-100 Holmers Farm Way, High Wycombe, Bucks, HP12 4EG, UK
Therapeutic indications
RISPERDAL CONSTA is indicated for the maintenance treatment of schizophrenia in patients currently stabilised with oral antipsychotics.
Posology and method of administration
Posology
Adults
Starting dose
For most patients the recommended dose is 25 mg intramuscular every two weeks. For those patients on a fixed dose of oral risperidone for two weeks or more, the following conversion scheme should be considered. Patients treated with a dosage of 4 mg or less oral risperidone should receive 25 mg RISPERDAL CONSTA, while patients treated with higher oral doses should be considered for the higher RISPERDAL CONSTA dose of 37.5 mg.
Where patients are not currently taking oral risperidone, the oral pre-treatment dosage should be considered when choosing the I.M. starting dose. The recommended starting dose is 25 mg RISPERDAL CONSTA every two weeks. Patients on higher dosages of the used oral antipsychotic should be considered for the higher RISPERDAL CONSTA dose of 37.5 mg.
Sufficient antipsychotic coverage with oral risperidone or the previous antipsychotic should be ensured during the three-week lag period following the first RISPERDAL CONSTA injection (see section 5.2).
RISPERDAL CONSTA should not be used in acute exacerbations of schizophrenia without ensuring sufficient antipsychotic coverage with oral risperidone or the previous antipsychotic during the three-week lag period following the first RISPERDAL CONSTA injection.
Maintenance dose
For most patients the recommended dose is 25 mg intramuscular every two weeks. Some patients may benefit from the higher doses of 37.5 mg or 50 mg. Upward dosage adjustment should not be made more frequently than every 4 weeks. The effect of this dose adjustment should not be anticipated earlier than 3 weeks after the first injection with the higher dose. No additional benefit was observed with 75 mg in clinical trials. Doses higher than 50 mg every 2 weeks are not recommended.
Elderly
No dose adjustment is required. The recommended dose is 25 mg intramuscularly every two weeks. Where patients are not currently taking oral risperidone, the recommended dose is 25 mg RISPERDAL CONSTA every two weeks. For those patients on a fixed dose of oral risperidone for two weeks or more, the following conversion scheme should be considered. Patients treated with a dosage of 4 mg or less oral risperidone should receive 25 mg RISPERDAL CONSTA, while patients treated with higher oral doses should be considered for the higher RISPERDAL CONSTA dose of 37.5 mg.
Sufficient antipsychotic coverage should be ensured during the three-week lag period following the first RISPERDAL CONSTA injection (see section 5.2). RISPERDAL CONSTA clinical data in elderly are limited. RISPERDAL CONSTA should be used with caution in elderly.
Hepatic and renal impairment
RISPERDAL CONSTA has not been studied in hepatically and renally impaired patients.
If hepatically or renally impaired patients require treatment with RISPERDAL CONSTA, a starting dose of 0.5 mg twice daily oral risperidone is recommended during the first week. The second week 1 mg twice daily or 2 mg once daily can be given. If an oral total daily dose of at least 2 mg is well tolerated, an injection of 25 mg RISPERDAL CONSTA can be administered every 2 weeks.
Sufficient antipsychotic coverage should be ensured during the three-week lag period following the first RISPERDAL CONSTA injection (see section 5.2).
Paediatric population
The safety and efficacy of RISPERDAL CONSTA in children below 18 years of age have not been established. No data are available.
Method of administration
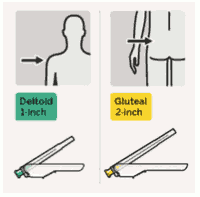
RISPERDAL CONSTA should be administered every two weeks by deep intramuscular deltoid or gluteal injection using the appropriate safety needle. For deltoid administration, use the 1-inch needle alternating injections between the two arms. For gluteal administration, use the 2-inch needle alternating injections between the two buttocks. Do not administer intravenously (see sections 4.4 and 6.6).
For instructions on reconstitution of the medicinal product before administration, see section 6.6.
Overdose
While overdose is less likely to occur with parenteral than with oral medicinal products, information pertaining to oral is presented.
Symptoms
In general, reported signs and symptoms have been those resulting from an exaggeration of the known pharmacological effects of risperidone. These include drowsiness and sedation, tachycardia and hypotension, and extrapyramidal symptoms. In overdose, QT prolongation and convulsions have been reported. Torsade de Pointes has been reported in association with combined overdose of oral RISPERDAL and paroxetine.
In case of acute overdose, the possibility of multiple drug involvement should be considered.
Treatment
Establish and maintain a clear airway and ensure adequate oxygenation and ventilation. Cardiovascular monitoring should commence immediately and should include continuous electrocardiographic monitoring to detect possible arrhythmias.
There is no specific antidote to RISPERDAL. Therefore appropriate supportive measures should be instituted. Hypotension and circulatory collapse should be treated with appropriate measures such as intravenous fluids and/or sympathomimetic agents. In case of severe extrapyramidal symptoms, anticholinergic medicinal product should be administered. Close medical supervision and monitoring should continue until the patient recovers.
Shelf life
Shelf life: 3 years at 2-8°C.
After reconstitution: Chemical and physical in-use stability has been demonstrated for 24 hours at 25°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 6 hours at 25°C, unless reconstitution has taken place in controlled and validated aseptic conditions.
Special precautions for storage
The entire dose pack should be stored in the refrigerator (2-8°C).
If refrigeration is unavailable, RISPERDAL CONSTA can be stored at temperatures not exceeding 25°C for no more than 7 days prior to administration.
Store in the original package in order to protect from light.
For storage conditions of the reconstituted medicinal product, see section 6.3.
Nature and contents of container
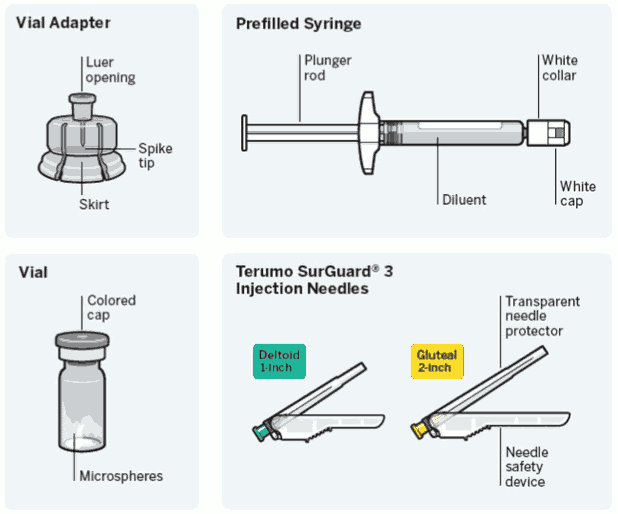
Needle-free vial access device
- One vial containing powder.
- One vial adapter for reconstitution.
- One prefilled syringe containing the solvent for RISPERDAL CONSTA.
- Two Terumo SurGuard3 needles for intramuscular injection (a 21G UTW 1-inch (0.8 mm × 25 mm) safety needle with needle protection device for deltoid administration and a 20G TW 2-inch (0.9 mm × 51 mm) safety needle with needle protection device for gluteal administration).
RISPERDAL CONSTA is available in packs containing 1 or 5 (bundled) packs.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
Important information
RISPERDAL CONSTA requires close attention to these step-by-step Instructions for Use to help ensure successful administration.
Use components provided: The components in this dose pack are specifically designed for use with RISPERDAL CONSTA. RISPERDAL CONSTA must be reconstituted only in the diluent supplied in the dose pack.
Do not substitute ANY components of the dose pack.
Do not store suspension after reconstitution.
Administer dose as soon as possible after reconstitution to avoid settling.
Proper dosing: The entire contents of the vial must be administered to ensure intended dose of RISPERDAL CONSTA is delivered.
Single-use device
Do not reuse: Medical devices require specific material characteristics to perform as intended. These characteristics have been verified for single use only. Any attempt to re-process the device for subsequent re-use may adversely affect the integrity of the device or lead to deterioration in performance.
Dose pack contents:
Step 1. Assemble components
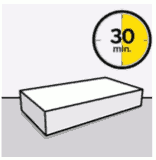
Take out dose pack:
Wait 30 minutes.
Remove dose pack from the refrigerator and allow to sit at room temperature for at least 30 minutes before reconstituting.
Do not warm any other way.
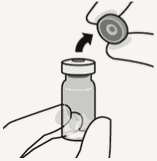
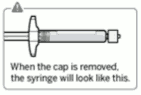
Connect vial adapter to vial:
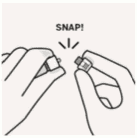
Remove cap from vial:
Flip off colored cap from vial.
Wipe top of the grey stopper with an alcohol swab.
Allow to air dry.
Do not remove grey rubber stopper.
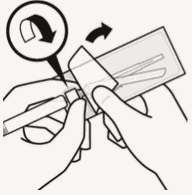
Prepare vial adapter:
Hold sterile blister as shown.
Peel back and remove paper backing.
Do not remove vial adapter from blister.
Do not touch spike tip at any time. This will result in contamination.
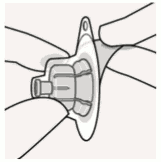
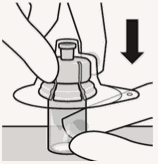
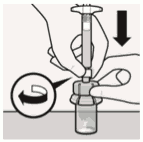
Connect vial adapter to vial:
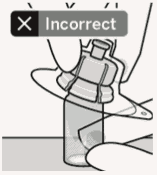
Place vial on a hard surface and hold by the base. Center vial adapter over the grey rubber stopper. Push vial adapter straight down onto vial top until it snaps securely into place.
Do not place vial adapter on at an angle or diluent may leak upon transfer to the vial.
Connect prefilled syringe to vial adapter:
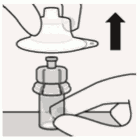
Remove sterile blister:
Remove vial adapter from sterile blister only when you are ready to remove thw white cap from the prefilled syringe.
Keep vial vertical to prevent leakage.
Hold base of vial and pull up on the sterile blister to remove.
Do not shake.
Do not touch exposed luer opening on vial adapter.
This will result in contamination.
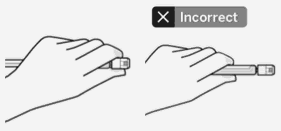
Use proper grip:
Hold by white collar at the tip of the syringe.
Do not hold syringe by the glass barrel during assembly.
Remove cap:
Holding the white collar, snap off the white cap.
Do not twist or cut off the white cap.
Do not touch syringe tip. This will result in contamination.
The broken-off cap can be discarded.
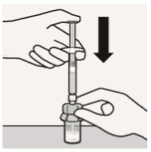
Connect syringe to vial adapter:
Hold vial adapter by skirt to keep stationary.
Hold syringe by white collar then insert tip into the luer opening of the vial adapter.
Do not hold the glass syringe barrel.
This may cause the white collar to loosen or detach.
Attach the syringe to the vial adapter with a firm clockwise twisting motion until it feels snug.
Do not over-tighten. Over-tightening may cause the syringe tip to break.
Step 2. Reconstitute microspheres
Inject diluent:
Inject entire amount of diluent from syringe into the vial.
Vial contents will noq be under pressure.
Keep holding the plumger rod down with the thumb.
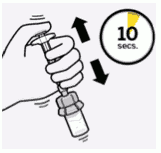
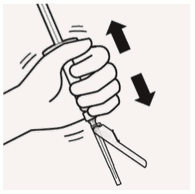
Suspend microspheres in diluent:
Continuing to hold down the plunger rod, shake vigorously for at least 10 seconds, as shown.
Check the suspension:
When properly mixed, the suspension appears uniform, thick and milky in color. Microspheres will be visible in the liquid.
Immediately proceed to the next step so suspension does not settle.
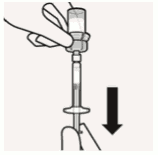
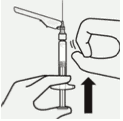
Transfer suspension to syringe:
Invert vial completely. Slowly pull plunger rod down to withdraw entire contents from the vial into the syringe.
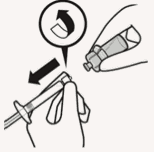
Remove vial adapter:
Hold white collar on the syringe and unscrew from vial adapter.
Tear section of the vial label at the perforation. Apply detached label to the syringe for identification purposes.
Discard both vial and vial adapter appropriately.
Step 3. Attach needle
Select appropriate needle:
Choose needle based on injection location (gluteal or deltoid).
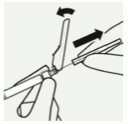
Attach needle:
Peel blister pouch open part way and use to grasp the base of the needle, as shown.
Holding the white collar on the syringe, attach syringe to needle luer connection with a firm clockwise twisting motion until snug.
Do not touch needle luer opening. This will result in contamination.
Resuspend microspheres:
Fully remove the blister pouch.
Just before injection, shake syringe vigorously again, as some settling will have occurred.
Step 4. Inject dose
Remove transparent needle protector:
Move the needle safety device back towards the syringe, as shown. Then hold white collar on syringe and carefully pull the transparent needle protector straight off.
Do not twist transparent needle protector, as the luer connection may loosen.
Remove air bubbles:
Hold syringe upright and tap gently to make any air bubbles rise to the top.
Slowly and carefully press plunger rod upward to remove air.
Inject:
Immediately inject entire contents of syringe intramuscularly (IM) into the gluteal or deltoid muscle of the patient.
Gluteal injection should be made into the upper-outer quadrant of the gluteal area.
Do not administer intravenously.
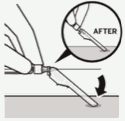
Secure needle in safety device:
Using one hand, place needle safety device at a 45 degree angle on a hard, flat surface. Press down with a firm, quick motion until needle is fully engaged in safety device.
Avoid needle stick injury:
Do not use two hands.
Do not intentionally disengage or mishandle the needle safety device.
Do not attempt to straighten the needle or engage the safety device if the needle is bent or damaged.
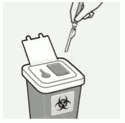
Properly dispose of needles:
Check to confirm needle safety device is fully engaged.
Discard in an approved sharps container.
Also discard the unused needle provided in the dose pack.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.