RYBELSUS Tablet Ref.[27432] Active ingredients: Semaglutide
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
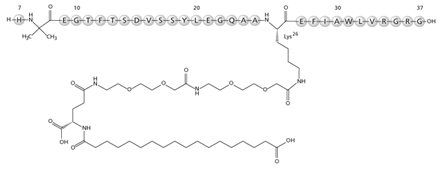
RYBELSUS tablets, for oral use, contain semaglutide, a GLP-1 receptor agonist. The peptide backbone is produced by yeast fermentation. The main protraction mechanism of semaglutide is albumin binding, facilitated by modification of position 26 lysine with a hydrophilic spacer and a C18 fatty di-acid. Furthermore, semaglutide is modified in position 8 to provide stabilization against degradation by the enzyme dipeptidyl-peptidase 4 (DPP-4). A minor modification was made in position 34 to ensure the attachment of only one fatty di-acid. The molecular formula is C187H291N45O59 and the molecular weight is 4113.58 g/mol.
Structural formula:
Semaglutide is a white to almost white hygroscopic powder. Each tablet of RYBELSUS contains 3 mg, 7 mg or 14 mg of semaglutide and the following inactive ingredients: magnesium stearate, microcrystalline cellulose, povidone and salcaprozate sodium (SNAC).
| Dosage Forms and Strengths |
|---|
|
RYBELSUS tablets are available as:
|
| How Supplied | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
RYBELSUS tablets are available as follows:
Manufactured by: Novo Nordisk A/S, DK-2880 Bagsvaerd, Denmark |
Drugs
| Drug | Countries | |
|---|---|---|
| RYBELSUS | Brazil, Canada, Estonia, Spain, Finland, France, Croatia, Ireland, Israel, Italy, Japan, Lithuania, Netherlands, Poland, Romania, Turkey, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.