RYBELSUS Tablet Ref.[27432] Active ingredients: Semaglutide
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
Semaglutide is a GLP-1 analogue with 94% sequence homology to human GLP-1. Semaglutide acts as a GLP-1 receptor agonist that selectively binds to and activates the GLP-1 receptor, the target for native GLP-1.
GLP-1 is a physiological hormone that has multiple actions on glucose, mediated by the GLP-1 receptors.
The principal mechanism of protraction resulting in the long half-life of semaglutide is albumin binding, which results in decreased renal clearance and protection from metabolic degradation. Furthermore, semaglutide is stabilized against degradation by the DPP-4 enzyme.
Semaglutide reduces blood glucose through a mechanism where it stimulates insulin secretion and lowers glucagon secretion, both in a glucose-dependent manner. Thus, when blood glucose is high, insulin secretion is stimulated and glucagon secretion is inhibited. The mechanism of blood glucose lowering also involves a minor delay in gastric emptying in the early postprandial phase.
12.2. Pharmacodynamics
All pharmacodynamic evaluations were performed after 12 weeks of treatment (including dose escalation) at steady state semaglutide injection 1 mg.
Fasting and Postprandial Glucose
Semaglutide reduces fasting and postprandial glucose concentrations. In patients with type 2 diabetes, treatment with semaglutide injection 1 mg resulted in reductions in glucose in terms of absolute change from baseline and relative reduction compared to placebo of 29 mg/dL (22%) for fasting glucose, 74 mg/dL (36%) for 2 hour postprandial glucose, and 30 mg/dL (22%) for mean 24 hour glucose concentration.
Insulin Secretion
Both first-and second-phase insulin secretion are increased in patients with type 2 diabetes treated with semaglutide compared with placebo.
Glucagon Secretion
Semaglutide lowers the fasting and postprandial glucagon concentrations.
Glucose dependent insulin and glucagon secretion
Semaglutide lowers high blood glucose concentrations by stimulating insulin secretion and lowering glucagon secretion in a glucose-dependent manner.
During induced hypoglycemia, semaglutide did not alter the counter regulatory responses of increased glucagon compared to placebo and did not impair the decrease of C-peptide in patients with type 2 diabetes.
Gastric emptying
Semaglutide causes a delay of early postprandial gastric emptying, thereby reducing the rate at which glucose appears in the circulation postprandially.
Cardiac electrophysiology (QTc)
The effect of subcutaneously administered semaglutide on cardiac repolarization was tested in a thorough QTc trial. At an average exposure level 4-fold higher than that of the maximum recommended dose of RYBELSUS, semaglutide does not prolong QTc intervals to any clinically relevant extent.
12.3. Pharmacokinetics
Absorption
Semaglutide is co-formulated with salcaprozate sodium which facilitates the absorption of semaglutide after oral administration. The absorption of semaglutide predominantly occurs in the stomach.
Population pharmacokinetics (PK) estimated semaglutide exposure to increase in a dose-proportional manner. In patients with type 2 diabetes, the mean population-PK estimated steady-state concentrations following once daily oral administration of 7 and 14 mg semaglutide were approximately 6.7 nmol/L and 14.6 nmol/L, respectively.
Following oral administration, maximum concentration of semaglutide is reached 1 hour post-dose. Steady-state exposure is achieved following 4-5 weeks administration.
Population-PK estimated absolute bioavailability of semaglutide to be approximately 0.4%-1%, following oral administration.
Distribution
The estimated volume of distribution of semaglutide following oral administration in healthy subjects is approximately 8 L. Semaglutide is extensively bound to plasma albumin (>99%).
Elimination
With an elimination half-life of approximately 1 week, semaglutide is present in the circulation for about 5 weeks after the last dose. The clearance of semaglutide following oral administration in healthy subjects is approximately 0.04 L/h.
Metabolism
The primary route of elimination for semaglutide is metabolism following proteolytic cleavage of the peptide backbone and sequential beta-oxidation of the fatty acid side chain.
Excretion
The primary excretion routes of semaglutide-related material are via the urine and feces. Approximately 3% of the absorbed dose is excreted in the urine as intact semaglutide.
Specific Populations
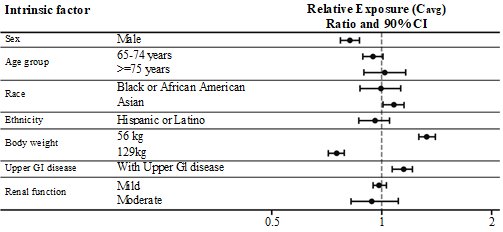
Based on a population pharmacokinetic analysis, age, sex, race, ethnicity, upper GI disease, and renal impairment do not have a clinically meaningful effect on the pharmacokinetics of semaglutide. The exposure of semaglutide decreases with an increase in body weight. However, RYBELSUS doses of 7 mg and 14 mg provide adequate systemic exposure over the body weight range of 40-188 kg evaluated in the clinical trials. The effects of intrinsic factors on the pharmacokinetics of semaglutide are shown in Figure 1.
Figure 1. Impact of intrinsic factors on semaglutide exposure:
Semaglutide exposure (Cavg) relative to reference subject profile: White, non-Hispanic or Latino female aged 18-64 years, with body weight of 85 kg, without upper GI disease or renal impairment, dosed 14 mg. Body weight categories (56 and 129 kg) represent the 5% and 95% percentiles in the dataset.
Abbreviations: Cavg: average semaglutide concentration. GI: gastrointestinal. CI: confidence interval.
Patients with Renal impairment
Renal impairment does not impact the pharmacokinetics of semaglutide in a clinically relevant manner. This was shown in a study with 10 consecutive days of once daily oral doses of semaglutide in patients with different degrees of renal impairment (mild, moderate, severe, end staged renal disease) compared with subjects with normal renal function. This was also shown for subjects with both type 2 diabetes and renal impairment based on data from clinical studies (Figure 1).
Patients with Hepatic impairment
Hepatic impairment does not have any impact on the exposure of semaglutide. The pharmacokinetics of semaglutide were evaluated in patients with different degrees of hepatic impairment (mild, moderate, severe) compared with subjects with normal hepatic function in a study with 10 consecutive days of once daily oral doses of semaglutide.
Patients with disease in the upper GI tract
Upper GI disease (chronic gastritis and/or gastroesophageal reflux disease) does not impact semaglutide pharmacokinetics in a clinically relevant manner. This was shown in a study in patients with type 2 diabetes with or without upper GI disease dosed for 10 consecutive days with once daily oral doses of semaglutide.
Pediatric Patients
Semaglutide has not been studied in pediatric patients.
Drug Interaction Studies
In vitro studies have shown very low potential for semaglutide to inhibit or induce CYP enzymes, and to inhibit drug transporters.
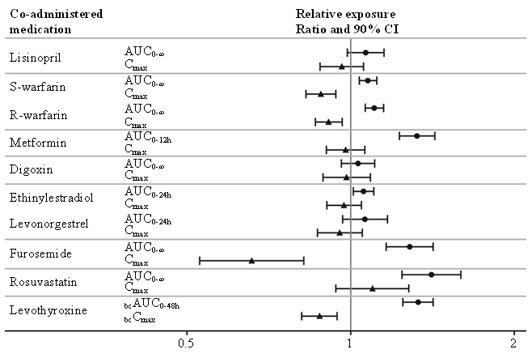
The delay of gastric emptying with semaglutide may influence the absorption of concomitantly administered oral medicinal products. Trials were conducted to study the potential effect of semaglutide on the absorption of oral medications taken with semaglutide administered orally at steady-state exposure.
No clinically relevant drug-drug interaction with semaglutide (Figure 2) was observed based on the evaluated medications. Total exposure (AUC) of thyroxine (adjusted for endogenous levels) was increased by 33% following administration of a single dose of levothyroxine 600 µg concurrently administered with semaglutide. Maximum exposure (Cmax) was unchanged [see Drug Interactions (7.2)].
Figure 2. Impact of semaglutide on the exposure of treatment with other oral medications:
Relative exposure in terms of AUC and Cmax for each medication when given with semaglutide compared to without semaglutide. Metformin and oral contraceptive drug (ethinylestradiol/levonorgestrel) were assessed at steady state. Effect on levothyroxine is measured as baseline corrected total T4 (thyroxine) concentration. Lisinopril, warfarin (S-warfarin/R-warfarin), digoxin, furosemide, rosuvastatin and levothyroxine were assessed after a single dose.
Abbreviations: AUC: area under the curve. Cmax: maximum concentration. CI: confidence interval.
No clinically relevant change in semaglutide exposure was observed when taken with omeprazole.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year carcinogenicity study in CD-1 mice, subcutaneous doses of 0.3, 1 and 3 mg/kg/day [9-, 33- and 113-fold the maximum recommended human dose (MRHD) of RYBELSUS 14 mg, based on AUC] were administered to the males, and 0.1, 0.3 and 1 mg/kg/day (3-, 9-, and 33-fold MRHD) were administered to the females. A statistically significant increase in thyroid C-cell adenomas and a numerical increase in C-cell carcinomas were observed in males and females at all dose levels (>3X human exposure).
In a 2-year carcinogenicity study in Sprague Dawley rats, subcutaneous doses of 0.0025, 0.01, 0.025 and 0.1 mg/kg/day were administered (below quantification, 0.8-, 1.8- and 11-fold the exposure at the MRHD). A statistically significant increase in thyroid C-cell adenomas was observed in males and females at all dose levels, and a statistically significant increase in thyroid C-cell carcinomas was observed in males at ≥0.01 mg/kg/day, at clinically relevant exposures.
Human relevance of thyroid C-cell tumors in rats is unknown and could not be determined by clinical studies or nonclinical studies [see Boxed Warning and Warnings and Precautions (5.1)].
Semaglutide was not mutagenic or clastogenic in a standard battery of genotoxicity tests (bacterial mutagenicity (Ames), human lymphocyte chromosome aberration, rat bone marrow micronucleus).
In a combined fertility and embryo-fetal development study in rats, subcutaneous doses of 0.01, 0.03 and 0.09 mg/kg/day (0.2-, 0.7- and 2.1-fold the MRHD) were administered to male and female rats. Males were dosed for 4 weeks prior to mating, and females were dosed for 2 weeks prior to mating and throughout organogenesis until Gestation Day 17. No effects were observed on male fertility. In females, an increase in estrus cycle length was observed at all dose levels, together with a small reduction in numbers of corpora lutea at ≥0.03 mg/kg/day. These effects were likely an adaptive response secondary to the pharmacological effect of semaglutide on food consumption and body weight.
13.2. Animal Toxicology and/or Pharmacology
Increase in lactate levels and decrease in glucose levels in the plasma and cerebrospinal fluid (CSF) were observed in mechanistic studies with SNAC in rats. Small but statistically significant increases in lactate levels (up to 2-fold) were observed in a few animals at approximately the clinical exposure. At higher exposures these findings were associated with moderate to marked adverse clinical signs (lethargy, abnormal respiration, ataxia, and reduced activity, body tone and reflexes) and marked decreases in plasma and CSF glucose levels. These findings are consistent with inhibition of cellular respiration and lead to mortality at SNAC concentrations ≥100-times the clinical Cmax.
14. Clinical Studies
14.1 Overview of Clinical Studies
RYBELSUS has been studied as monotherapy and in combination with metformin, sulfonylureas, sodium-glucose co-transporter-2 (SGLT-2) inhibitors, insulins, and thiazolidinediones in patients with type 2 diabetes. The efficacy of RYBELSUS was compared with placebo, empagliflozin, sitagliptin, and liraglutide. RYBELSUS has also been studied in patients with type 2 diabetes with mild and moderate renal impairment.
In patients with type 2 diabetes, RYBELSUS produced clinically significant reduction from baseline in HbA1c compared with placebo.
The efficacy of RYBELSUS was not impacted by baseline age, gender, race, ethnicity, BMI, body weight, diabetes duration and level of renal impairment.
14.2 Monotherapy Use of RYBELSUS in Patients with Type 2 Diabetes Mellitus
In a 26-week double-blind trial (NCT02906930), 703 patients with type 2 diabetes inadequately controlled with diet and exercise were randomized to RYBELSUS 3 mg, RYBELSUS 7 mg or RYBELSUS 14 mg once daily or placebo. Patients had a mean age of 55 years and 51% were men. The mean duration of type 2 diabetes was 3.5 years, and the mean BMI was 32 kg/m². Overall, 75% were White, 5% were Black or African American, and 17% were Asian; 26% identified as Hispanic or Latino ethnicity.
Monotherapy with RYBELSUS 7 mg and RYBELSUS 14 mg once daily for 26 weeks resulted in a statistically significant reduction in HbA1c compared with placebo (see Table 3).
Table 3. Results at Week 26 in a Trial of RYBELSUS as Monotherapy in Adult Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Diet and Exercise:
| Placebo | RYBELSUS7 mg | RYBELSUS14 mg | |
|---|---|---|---|
| Intent-to-Treat (ITT) Population (N)a | 178 | 175 | 175 |
| HbA1c (%) | |||
| Baseline (mean) | 7.9 | 8.0 | 8.0 |
| Change at week 26b | -0.3 | -1.2 | -1.4 |
| Difference from placebob [95% CI] | −0.9 [−1.1; −0.6]c | −1.1 [−1.3; −0.9]c | |
| Patients (%) achieving HbA1c <7% | 31 | 69 | 77 |
| FPG (mg/dL) | |||
| Baseline (mean) | 160 | 162 | 158 |
| Change at week 26b | -3 | -28 | -33 |
a The intent-to-treat population includes all randomized patients. At week 26, the primary HbA1c endpoint was missing for 5.6%, 8.6% and 8.6% of patients randomized to placebo, RYBELSUS 7 mg and RYBELSUS 14 mg, respectively. Missing data were imputed by a pattern mixture model using multiple imputation (MI). Pattern was defined by randomized treatment and treatment status at week 26. During the trial, additional anti-diabetic medication was initiated as an add on to randomized treatment by 15%, 2% and 1% of patients randomized to placebo, RYBELSUS 7 mg and RYBELSUS 14 mg, respectively.
b Estimated using an ANCOVA model based on data irrespectively of discontinuation of trial product or initiation of rescue medication adjusted for baseline value and region.
c p<0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
The mean baseline body weight was 88.6 kg, 89.0 kg and 88.1 kg in the placebo, RYBELSUS 7 mg, and RYBELSUS 14 mg arms, respectively. The mean changes from baseline to week 26 were -1.4 kg, -2.3 kg and -3.7 kg in the placebo, RYBELSUS 7 mg, and RYBELSUS 14 mg arms, respectively. The difference from placebo (95% CI) for RYBELSUS 7 mg was -0.9 kg (-1.9, 0.1) and for RYBELSUS 14 mg was -2.3 kg (-3.1, -1.5).
14.3 Combination Therapy Use of RYBELSUS in Patients with Type 2 Diabetes Mellitus
Combination with metformin
In a 26-week trial (NCT02863328), 822 patients with type 2 diabetes were randomized to RYBELSUS 14 mg once daily or empagliflozin 25 mg once daily, all in combination with metformin. Patients had a mean age of 58 years and 50% were men. The mean duration of type 2 diabetes was 7.4 years, and the mean BMI was 33 kg/m². Overall, 86% were White, 7% were Black or African American, and 6% were Asian; 24% identified as Hispanic or Latino ethnicity.
Treatment with RYBELSUS 14 mg once daily for 26 weeks resulted in a statistically significant reduction in HbA1c compared to empagliflozin 25 mg once daily (see Table 4).
Table 4. Results at Week 26 in a Trial of RYBELSUS Compared to Empagliflozin in Adult Patients with Type 2 Diabetes Mellitus In Combination with Metformin:
| RYBELSUS 14 mg | Empagliflozin 2 mg | |
|---|---|---|
| Intent-to-Treat (ITT) Population (N) a | 411 | 410 |
| HbA1c (%) | ||
| Baseline (mean) | 8.1 | 8.1 |
| Change at week 26b | -1.3 | -0.9 |
| Difference from empagliflozinb [95% CI] | -0.4 [-0.6, -0.3] c | |
| Patients (%) achieving HbA1c <7% | 67 | 40 |
| FPG (mg/dL) | ||
| Baseline (mean) | 172 | 174 |
| Change at week 26b | -36 | -36 |
a The intent-to-treat population includes all randomized patients. At week 26, the primary HbA1c endpoint was missing for 4.6% and 3.7% of patients randomized to RYBELSUS 14 mg and empagliflozin 25 mg, respectively. Missing data were imputed by a pattern mixture model using multiple imputation (MI). Pattern was defined by randomized treatment and treatment status at week 26. During the trial, additional anti-diabetic medication was initiated as an add on to randomized treatment by 1.9% and 1.2% of patients randomized to RYBELSUS 14 mg and empagliflozin 25 mg, respectively.
b Estimated using an ANCOVA based on data irrespectively of discontinuation of trial product or initiation of rescue medication adjusted for baseline value and region.
c p<0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
The mean baseline body weight was 91.9 kg and 91.3 kg in the RYBELSUS 14 mg and empagliflozin 25 mg arms, respectively. The mean changes from baseline to week 26 were -3.8 kg and -3.7 kg in the RYBELSUS 14 mg and empagliflozin 25 mg arms, respectively. The difference from empagliflozin (95% CI) for RYBELSUS 14 mg was -0.1 kg (-0.7, 0.5).
Combination with metformin or metformin with sulfonylurea
In a 26-week, double-blind trial (NCT02607865), 1864 patients with type 2 diabetes on metformin alone or metformin with sulfonylurea were randomized to RYBELSUS 3 mg, RYBELSUS 7 mg, RYBELSUS 14 mg or sitagliptin 100 mg once daily. Patients had a mean age of 58 years and 53% were men. The mean duration of type 2 diabetes was 8.6 years, and the mean BMI was 32 kg/m². Overall, 71% were White, 9% were Black or African American, and 13% were Asian; 17% identified as Hispanic or Latino ethnicity.
Treatment with RYBELSUS 7 mg and RYBELSUS 14 mg once daily for 26 weeks resulted in a statistically significant reduction in HbA1c compared to sitagliptin 100 mg once daily (see Table 5).
Table 5. Results at Week 26 in a Trial of RYBELSUS Compared to Sitagliptin 100 mg Once daily in Adult Patients with Type 2 Diabetes Mellitus In Combination with Metformin or Metformin with Sulfonylurea:
| RYBELSUS 7 mg | RYBELSUS 14 mg | Sitagliptin 100 mg | |
|---|---|---|---|
| Intent-to-Treat (ITT) Population (N)a | 465 | 465 | 467 |
| HbA1c (%) | |||
| Baseline (mean) | 8.4 | 8.3 | 8.3 |
| Change at week 26b | -1.0 | -1.3 | -0.8 |
| Difference from sitagliptinb [95% CI] | -0.3 [-0.4; -0.1]c | -0.5 [-0.6; -0.4]c | |
| Patients (%) achieving HbA1c <7% | 44 | 56 | 32 |
| FPG (mg/dL) | |||
| Baseline (mean) | 170 | 168 | 172 |
| Change at week 26b | -21 | -31 | -15 |
a The intent-to-treat population includes all randomized patients. At week 26, the primary HbA1c endpoint was missing for 5.8%, 6.2% and 4.5% of patients randomized to RYBELSUS 7 mg, RYBELSUS 14 mg and sitagliptin 100 mg, respectively. Missing values were imputed by a pattern mixture model using multiple imputation (MI). Pattern was defined by randomized treatment and treatment status at week 26. During the trial, additional anti-diabetic medication was initiated as an add on to randomized treatment by 2.4%, 1.1% and 2.8% of patients randomized to RYBELSUS 7 mg, RYBELSUS 14 mg and sitagliptin 100 mg, respectively.
b Estimated using an ANCOVA based on data irrespectively of discontinuation of trial product or initiation of rescue medication adjusted for baseline value, background medication and region.
c p<0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
The mean baseline body weight was 91.3 kg, 91.2 kg and 90.9 kg in the RYBELSUS 7 mg, RYBELSUS 14 mg and sitagliptin 100 mg arms, respectively. The mean changes from baseline to week 26 were -2.2 kg, -3.1 kg and -0.6 kg in the RYBELSUS 7 mg, RYBELSUS 14 mg and sitagliptin 100 mg arms, respectively. The difference from sitagliptin (95% CI) for RYBELSUS 7 mg was -1.6 kg (-2.0, -1.1) and RYBELSUS 14 mg was -2.5 kg (-3.0, -2.0).
Combination with metformin or metformin with SGLT-2 inhibitors
In a 26-week, double-blind, double-dummy trial (NCT02863419), 711 patients with type 2 diabetes on metformin alone or metformin with SGLT-2 inhibitors were randomized to RYBELSUS 14 mg once daily, liraglutide 1.8 mg s.c. injection once daily or placebo. Patients had a mean age of 56 years and 52% were men. The mean duration of type 2 diabetes was 7.6 years, and the mean BMI was 33 kg/m². Overall, 73% were White, 4% were Black or African American, and 13% were Asian; 6% identified as Hispanic or Latino ethnicity.
Treatment with RYBELSUS 14 mg once daily for 26 weeks resulted in statistically significant reductions in HbA1c compared to placebo. Treatment with RYBELSUS 14 mg once daily for 26 weeks resulted in non-inferior reductions in HbA1c compared to liraglutide 1.8 mg (see Table 6).
Table 6. Results at Week 26 in a Trial of RYBELSUS Compared to Liraglutide and Placebo in Adult Patients with Type 2 Diabetes Mellitus In Combination with Metformin or Metformin with SGLT-2i
| Placebo | Liraglutide 1.8 mg | RYBELSUS 14 mg | |
|---|---|---|---|
| Intent-to-Treat (ITT) Population (N)a | 142 | 284 | 285 |
| HbA1c (%) | |||
| Baseline (mean) | 7.9 | 8.0 | 8.0 |
| Change at week 26b | -0.2 | -1.1 | -1. 2 |
| Difference from placebob [95% CI] | -1.1 [-1.2; -0.9]c | ||
| Difference from liraglutideb [95% CI] | -0.1 [-0.3; 0.0] | ||
| Patients (%) achieving HbA1c <7% | 14 | 62 | 68 |
| FPG (mg/dL) | |||
| Baseline (mean) | 167 | 168 | 167 |
| Change at week 26b | -7 | -34 | -36 |
a The intent-to-treat population includes all randomized patients. At week 26, the primary HbA1c endpoint was missing for 5.6%, 4.2% and 2.5% of patients randomized to placebo, liraglutide 1.8 mg and RYBELSUS 14 mg, respectively. Missing values were imputed by a pattern mixture model using multiple imputation (MI). Pattern was defined by randomized treatment and treatment status at week 26. During the trial, additional anti-diabetic medication was initiated as an add on to randomized treatment by 7.7%, 3.2% and 3.5% of patients randomized to placebo, liraglutide 1.8 mg and RYBELSUS 14 mg respectively.
b Estimated using an ANCOVA based on data irrespectively of discontinuation of trial product or initiation of rescue medication adjusted for baseline value, background medication and region.
c p<0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
The mean baseline body weight was 93.2 kg, 95.5 kg and 92.9 kg in the placebo, liraglutide 1.8 mg, and RYBELSUS 14 mg arms, respectively. The mean changes from baseline to week 26 were -0.5 kg, -3.1 kg and -4.4 kg in the placebo, liraglutide 1.8 mg, and RYBELSUS 14 mg arms, respectively. The difference from placebo (95% CI) for RYBELSUS 14 mg was -3.8 kg (-4.7, -3.0). The difference from liraglutide 1.8 mg for RYBELSUS 14 mg was -1.2 (-1.9, -0.6).
Combination in patients with Type 2 Diabetes Mellitus and Moderate Renal Impairment with Metformin alone, Sulfonylurea alone, Basal Insulin alone, or Metformin in Combination with either Sulfonylurea or Basal Insulin
In a 26-week, double-blind trial (NCT02827708), 324 patients with moderate renal impairment (eGFRCKD-EPI 30−59 mL/min/1.73 m²) were randomized to RYBELSUS 14 mg or placebo once daily. RYBELSUS was added to the patient's stable pre-trial antidiabetic regimen. The insulin dose was reduced by 20% at randomization for patients on basal insulin. Dose reduction of insulin and sulfonylurea was allowed in case of hypoglycemia; up titration of insulin was allowed but not beyond the pre-trial dose.
Patients had a mean age of 70 years and 48% were men. The mean duration of type 2 diabetes was 14 years, and the mean BMI was 32 kg/m². Overall, 96% were White, 4% were Black or African American, and 0.3% were Asian; 6.5% identified as Hispanic or Latino ethnicity. 39.5% of patients had an eGFR value of 30 to 44 mL/min/1.73 m².
Treatment with RYBELSUS 14 mg once daily for 26 weeks resulted in a statistically significant reduction in HbA1c from baseline compared to placebo (see Table 7).
Table 7. Results at Week 26 in a Trial of RYBELSUS Compared to Placebo in Patients With Moderate Renal Impairment:
| Placebo | RYBELSUS 14 mg | |
|---|---|---|
| Intent-to-Treat (ITT) Population (N) a | 161 | 163 |
| HbA1c (%) | ||
| Baseline (mean) | 7.9 | 8.0 |
| Change at week 26b | -0.2 | -1.0 |
| Difference from placebob [95% CI] | -0.8[-1.0; -0.6]c | |
| Patients (%) achieving HbA1c <7% | 23 | 58 |
| FPG (mg/dL) | ||
| Baseline (mean) | 164 | 164 |
| Change at week 26b | -7 | -28 |
a The intent-to-treat population includes all randomized patients including patients on rescue medication. At week 26, the primary HbA1c endpoint was missing for 3.7% and 5.5% of patients randomized to placebo and RYBELSUS 14 mg, respectively. Missing values were imputed by a pattern mixture model using multiple imputation (MI). Pattern was defined by randomized treatment and treatment status at week 26. During the trial, additional anti-diabetic medication was initiated as an add on to randomized treatment by 10% and 4.3% of patients randomized to placebo and RYBELSUS 14 mg, respectively.
b Estimated using an ANCOVA based on data irrespectively of discontinuation of trial product or initiation of rescue medication adjusted for baseline value, background medication, renal status and region.
c p<0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
The mean baseline body weight was 90.4 kg and 91.3 kg in the placebo and RYBELSUS 14 mg arms, respectively. The mean changes from baseline to week 26 were -0.9 kg and -3.4 kg in the placebo and RYBELSUS 14 mg arms, respectively. The difference from placebo (95% CI) for RYBELSUS 14 mg was -2.5 kg (-3.2, -1.8).
Combination with Insulin with or without Metformin
In a 26-week double blind trial (NCT03021187), 731 patients with type 2 diabetes inadequately controlled on insulin (basal, basal/bolus or premixed) with or without metformin, were randomized to RYBELSUS 3 mg, 7 mg and 14 mg once daily or placebo once daily. All patients reduced their insulin dose by 20% at randomization to reduce the risk of hypoglycemia. Patients were allowed to increase the insulin dose only up to the starting insulin dose prior to randomization.
Patients had a mean age of 61 years and 54% were men. The mean duration of type 2 diabetes was 15 years, and the mean BMI was 31 kg/m². Overall, 51% were White, 7% were Black or African American, and 36% were Asian; 13% identified as Hispanic or Latino ethnicity.
Treatment with RYBELSUS 7 mg and 14 mg once daily for 26 weeks resulted in a statistically significant reduction in HbA1c from baseline compared to placebo once daily (see Table 8).
Table 8. Results at Week 26 in a Trial of RYBELSUS Compared to Placebo in Adult Patients with Type 2 Diabetes Mellitus In Combination with Insulin alone or with Metformin:
| Placebo | RYBELSUS 7 mg | RYBELSUS 14 mg | |
|---|---|---|---|
| Intent-to-Treat (ITT) Population (N)a | 184 | 182 | 181 |
| HbA1c (%) | |||
| Baseline (mean) | 8.2 | 8.2 | 8.2 |
| Change at week 26b | -0.1 | -0.9 | -1.3 |
| Difference from placebob [95% CI] | -0.9[-1.1; -0.7]c | -1.2[-1.4; -1.0]c | |
| Patients (%) achieving HbA1c <7% | 7 | 43 | 58 |
| FPG (mg/dL) | |||
| Baseline (mean) | 150 | 153 | 150 |
| Change at week 26b | 5 | -20 | -24 |
a The intent-to-treat population includes all randomized patients. At week 26, the primary HbA1c endpoint was missing for 4.3%, 4.4%, and 4.4% of patients randomized to placebo, RYBELSUS 7 mg and RYBELSUS 14 mg, respectively. Missing values were imputed by a pattern mixture model using multiple imputation (MI). Pattern was defined by randomized treatment and treatment status at week 26. During the trial, additional anti-diabetic medication was initiated as an add on to randomized treatment by 4.9%, 1.1 % and 2.2% of patients randomized to placebo, RYBELSUS 7 mg and RYBELSUS 14 mg, respectively.
b Estimated using an ANCOVA based on data irrespectively of discontinuation of trial product or initiation of rescue medication adjusted for baseline value, background medication and region.
c p<0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
The mean baseline body weight was 86.0 kg, 87.1 kg and 84.6 kg in the placebo, RYBELSUS 7 mg, and RYBELSUS 14 mg arms, respectively. The mean changes from baseline to week 26 were -0.4 kg, -2.4 kg and -3.7 kg in the placebo, RYBELSUS 7 mg, and RYBELSUS 14 mg arms, respectively. The difference from placebo (95% CI) for RYBELSUS 7 mg was -2.0 kg (-3.0, -1.0), and for RYBELSUS 14 mg was -3.3 kg (-4.2, -2.3).
14.4 Cardiovascular Outcomes Trial in Patients with Type 2 Diabetes Mellitus and Cardiovascular Disease
PIONEER 6 (NCT02692716) was a multi-center, multi-national, placebo-controlled, double-blind trial. In this trial, 3,183 patients with inadequately controlled type 2 diabetes and atherosclerotic cardiovascular disease were randomized to RYBELSUS 14 mg once daily or placebo for a median observation time of 16 months. The trial compared the risk of a Major Adverse Cardiovascular Event (MACE) between RYBELSUS 14 mg and placebo when these were added to and used concomitantly with standard of care treatments for diabetes and cardiovascular disease. The primary endpoint, MACE, was the time to first occurrence of a three-part composite outcome which included cardiovascular death, non-fatal myocardial infarction and non-fatal stroke.
Patients eligible to enter the trial were; 50 years of age or older and had established, stable, cardiovascular, cerebrovascular, peripheral artery disease, chronic kidney disease or NYHA class II and III heart failure or were 60 years of age or older and had other specified risk factors for cardiovascular disease. In total, 1,797 patients (56.5%) had established cardiovascular disease without chronic kidney disease, 354 patients (11.1%) had chronic kidney disease only, and 544 patients (17.1%) had both cardiovascular disease and kidney disease; 488 patients (15.3%) had cardiovascular risk factors without established cardiovascular disease or chronic kidney disease. The mean age at baseline was 66 years, and 68% were men. The mean duration of diabetes was 14.9 years, and mean BMI was 32 kg/m². Overall, 72% were White, 6% were Black or African American, and 20% were Asian; 16% identified as Hispanic or Latino ethnicity. Concomitant diseases of patients in this trial included, but were not limited to, heart failure (12%), history of ischemic stroke (8%) and history of a myocardial infarction (36%). In total, 99.7% of the patients completed the trial and the vital status was known at the end of the trial for 100%.
For the primary analysis, a Cox proportional hazards model was used to test for non-inferiority of RYBELSUS 14 mg to placebo for time to first MACE using a risk margin of 1.3. Type-1 error was controlled across multiple tests using a hierarchical testing strategy. Non‑inferiority to placebo was established, with a hazard ratio equal to 0.79 (95% CI: 0.57, 1.11) over the median observation time of 16-months. The proportion of patients who experienced at least one MACE was 3.8% (61/1591) for RYBELSUS 14 mg and 4.8% (76/1592) for placebo.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.